Fill Out Your 227 Form
The DHHS Form 227 serves as an essential application for re-registration under the North Carolina Controlled Substances Act of 1971, designed for various entities that engage in the handling of controlled substances. This form collects crucial information, such as the applicant's name, mailing address, and contact details, along with their DEA number. It delineates the type of business activity being conducted—whether as a manufacturer, distributor, researcher, analytical laboratory, or dog handler—each of which incurs a specific fee. The form also requires applicants to indicate which drug schedules they intend to handle, ranging from Schedule I to Schedule VI, ensuring compliance with state and federal regulations. Critical questions address the applicant’s legal standing and history concerning controlled substances, aiming to ascertain any past convictions or registration issues that may impact their current application. In summary, Form 227 is a comprehensive document that streamlines the re-registration process while safeguarding public health and maintaining regulatory oversight.
227 Example
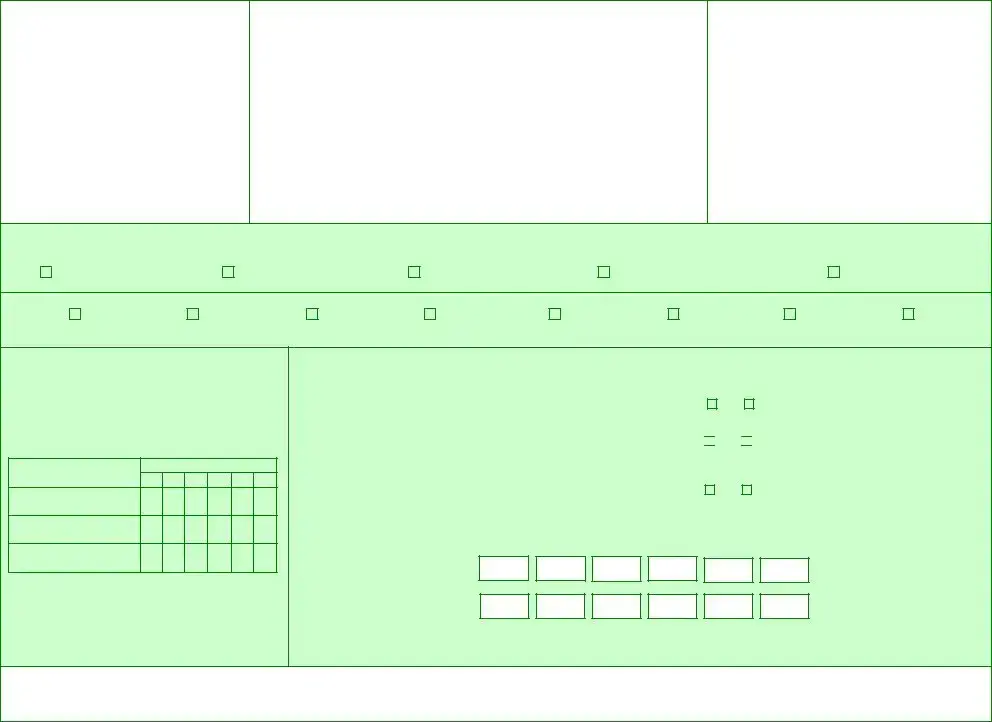
FORM DHHS 227
Application for Reregistration
under
N.C. Controlled Substances Act
of 1971
DHHS Registration No. ___________________
DEA No. ___________________
Please print or type all entries
____________________________________________________
Name of Applicant (Facility Name)
_________________________________________________________
Mailing Address
_________________________________________________________
Location
_________________________________________________________
TownCountyStateZip
Applicant Telephone: ___________________________________________
Area Code
Point of Contact Name: ________________________
Point of Contact Telephone: _____________________
RETAIN COPY
Mail Application to:
Department of Health and Human Services
Controller’s
2025 Mail Service Center
Raleigh, North Carolina
Telephone: (919)
REGISTRATION CLASSIFICATION: SUBMIT CHECK OR MONEY ORDER PAYABLE TO: SUBSTANCE ABUSE DRUG REGULATORY
1. Business Activity: (Check one only)
A
Manufacturer $600 |
B |
Distributor $500 |
C |
Researcher $125 |
D |
Analytical Laboratory $100 |
E |
Dog Handler $150
2.Drug Schedules: (Check all applicable)
Schedule I |
Schedule II |
|
Narcotic |
Schedule IIN
Schedule III
Narcotic
Schedule IIIN
Schedule IV
Schedule V
Schedule VI
3.Registration as a manufacturer conveys distribution privileges only as those substances manufactured.
Manufacturers (Item 1A, Business Activity) check schedules applicable to any category in the boxes below:
|
|
Schedules |
|
|
|
I |
II |
III |
IV |
V |
VI |
Bulk Manufacturer |
|
|
|
|
|
|
|
|
|
|
|
Dosage Form |
|
|
|
|
|
Manufacturer |
|
|
|
|
|
4.ALL APPLICANTS MUST ANSWER THE FOLLOWING:
(a)Are you currently authorized to manufacture, distribute, dispense, prescribe, conduct research, or otherwise handle the controlled substances in the schedules for which you applying under the laws of North Carolina or the Federal Government?
Yes |
No |
(b)Has the applicant been convicted of a felony under State or Federal law relating to the manufacture, possession, distribution, or dispensing of controlled substances?

 Yes
Yes 
 No
No
(c)Has any previous registration held by the applicant, corporation, firm, partner, or officer of applicant under Federal CSA or NCCSA been surrendered, revoked, suspended, denied, or is it pending such action?
Yes |
No |
If YES to b and/or c, attach a letter setting forth the circumstances of such action.
5. Drug code numbers must coincide with the schedules requested, listed below are the drug code requirements for each business activity:
Analytic Lab – Not Required To List Drug Codes
Distributor – Schedule I
Researcher – Schedule I, II, III, IV, V and VI
Manufacturer – Schedule I, II, III, IIIN
IF ADDITIONAL SPACE IS REQUIRED, USE A SEPARATE SHEET AND RETURN WITH APPLICATION
AUTHORIZED INDIVIDUAL |
|
|
|
__________________ |
________________________________ |
_________________________________ |
______________________________ |
Date |
Print or Type Name |
Signature |
Official Title |
Form Characteristics
| Fact Name | Description |
|---|---|
| Purpose | The DHHS 227 form is an application for re-registration under the North Carolina Controlled Substances Act of 1971. It ensures that facilities handling controlled substances comply with state regulations. |
| Submission Details | Applicants must mail the completed form to the Department of Health and Human Services in Raleigh, North Carolina. A copy should be retained for personal records. |
| Registration Classification | Applicants must select their business activity classification, such as Manufacturer or Distributor, with respective fees ranging from $100 to $600. |
| Governing Laws | The form is governed by the North Carolina Controlled Substances Act (NCCSA) and the Federal Controlled Substances Act (CSA). |
Guidelines on Utilizing 227
Completing the DHHS 227 form is a crucial step in maintaining compliance under North Carolina's Controlled Substances Act. It's important to provide accurate information to ensure your application is processed smoothly. Follow these steps carefully to fill out the form.
- Obtain the DHHS 227 form from the appropriate source.
- Enter your DHHS Registration No. and DEA No. in the designated fields.
- Print or type the Name of Applicant (Facility Name) clearly in the space provided.
- Fill in the Mailing Address with your complete address.
- Provide the Location details, including the town, county, state, and zip code.
- Enter the Applicant Telephone number, including the area code.
- List the Point of Contact Name and their Telephone number.
- Retain a copy of the completed application for your records.
- Mail the application to:
Department of Health and Human Services
Controller’s Office - Accounts Receivable
2025 Mail Service Center
Raleigh, North Carolina 27699-2025
Telephone: (919) 733-1765. - Check one box under Business Activity to indicate your type of business and the corresponding fee:
- A Manufacturer $600
- B Distributor $500
- C Researcher $125
- D Analytical Laboratory $100
- E Dog Handler $150
- Check all applicable Drug Schedules from the list provided.
- If applicable, check the relevant boxes under section 3 regarding distribution privileges for manufacturers.
- Answer all questions in section 4, indicating “Yes” or “No” as appropriate:
- (a) Authorization status for handling controlled substances.
- (b) Any felony convictions related to controlled substances.
- (c) Status of previous registrations.
- If you answered "Yes" to questions b and/or c, attach a letter explaining the circumstances.
- Ensure you list the appropriate drug code numbers according to your business activity in section 5.
- Designate an Authorized Individual by printing or typing their name, signature, and official title.
- Date the form at the bottom.
After completing the form, review all entries to ensure accuracy before sending it. This careful process will help in obtaining the necessary registration without unnecessary delays.
What You Should Know About This Form
What is Form DHHS 227?
Form DHHS 227 is the Application for Reregistration under the North Carolina Controlled Substances Act of 1971. It is used by facilities that wish to continue or renew their registration to handle controlled substances within North Carolina. This form collects necessary information related to the applicant and their business activities, ensuring compliance with state and federal laws.
Who needs to fill out this form?
This form must be filled out by any facility that is currently registered or wishes to register to manufacture, distribute, dispense, or conduct research with controlled substances in North Carolina. This includes manufacturers, distributors, researchers, and other related entities that handle these substances as defined by law.
What information is required on the form?
The form requires various types of information about the applicant. Key sections include the applicant's name, mailing address, and DEA number. Additionally, the form requires the applicant to indicate their business activity, applicable drug schedules, a declaration of past offenses related to controlled substances, and details about their authorized individuals. Ensuring that this information is accurate is crucial for successful reregistration.
How is the registration fee determined?
The registration fee varies based on the type of business activity. For example, a manufacturer pays $600, while a distributor pays $500. Other categories have different fees, such as $125 for researchers and $100 for analytical laboratories. Applicants must check the box that corresponds to their business activity and submit the appropriate fee with their application.
What happens if there has been a felony conviction?
If the applicant has been convicted of a felony relating to controlled substances, they must answer “yes” to the corresponding question on the form. In this case, attaching a letter that explains the circumstances surrounding the conviction will be necessary. This information will be reviewed as part of the evaluation process of the application.
Where should the completed application be mailed?
The completed Form DHHS 227 along with the payment should be mailed to the Department of Health and Human Services at the address listed on the form: Controller’s Office-Accounts Receivable, 2025 Mail Service Center, Raleigh, North Carolina 27699-2025. It is important to keep a copy of the application for your records.
What if I need additional space to provide information?
If additional space is needed to provide answers or details, applicants are encouraged to use a separate sheet and attach it to the application. This ensures that all necessary information is included without making the form cluttered or difficult to read.
What should I do if my previous registration was revoked or suspended?
In such cases, applicants must indicate “yes” to the relevant question on the form. A detailed letter explaining the circumstances surrounding the revocation or suspension of the previous registration should be attached. This transparency is critical for the review process and for addressing potential concerns regarding the new application.
What is the significance of the point of contact?
The point of contact is an individual designated by the applicant as the primary communicator for matters related to the application. Including accurate contact information for this individual ensures that any questions or follow-up regarding the application can be handled promptly and effectively.
Common mistakes
Filling out the DHHS 227 form can be straightforward, but many applicants make common mistakes that can delay or complicate their application process. Understanding these errors can help ensure a smoother submission.
One frequent mistake is failing to provide complete information. The form requires various details, such as the Name of Applicant and Mailing Address. Omitting any of this essential information can lead to processing delays. Ensure each section is filled out accurately and completely.
Another common error involves the selection of the Business Activity. Applicants might check multiple boxes instead of selecting only one. This section is designed for a single choice, and choosing more than one can create confusion about the application’s intent.
Many applicants also overlook the importance of checking the applicable Drug Schedules. It’s crucial to mark all that apply; leaving a box unchecked for a relevant schedule can result in an incomplete application. Pay close attention to the schedules of controlled substances that align with your business activity.
Failure to respond to the mandatory questions can also be problematic. Section 4 requires answers regarding prior felony convictions and historic registrations. Applicants often skip these questions, assuming they are optional. Providing honest and complete responses is essential to avoid future issues.
Another mistake is inadequate justification when answering "Yes" to questions in Section 4(b) or (c). If you answer yes, it’s necessary to attach a detailed letter explaining the circumstances surrounding any past legal issues. Omitting this documentation can lead to a rejection of your application.
Many applicants forget to include their signatures or provide a printed name and official title in the designated sections. Missing signatures can render the application invalid, causing further delays in obtaining registration.
Applicants often neglect to keep a copy of the completed application for their records. This can be problematic if questions arise later about the information provided. Retaining a copy helps in tracking your submission and provides a reference in case of discrepancies.
Finally, some individuals may not follow up on their application after submission. It is important to remember the contact details provided on the form. Checking in can clarify any outstanding issues and ensure the application is progressing smoothly.
Documents used along the form
Alongside the DHHS 227 form, various other forms and documents are relevant during the registration process for handling controlled substances. These documents support the application and help ensure compliance with federal and state regulations. Below are five commonly used forms and documents.
- Form DHHS 224: This is the application for a new registration with the Drug Enforcement Administration (DEA). Facilities seeking to manufacture or distribute controlled substances must complete this form to receive a DEA registration number, which is essential for lawful operations.
- Form DHHS 223: This form is used to register for a Controlled Substances Registration in North Carolina. It includes information about the applicant's qualifications and facilitates the evaluation of compliance with state regulations. A valid registration is required to conduct business with controlled substances.
- Form DEA 106: In the event of the theft or significant loss of controlled substances, applicants must submit this form to report the incident to the DEA. This action is crucial for tracking substances and maintaining security within the controlled substances framework.
- Form DEA 41: This document is for the disposal of controlled substances. When a facility needs to destroy expired or unwanted inventory, this form must be completed to ensure the process follows legal guidelines. Proper disposal is vital to prevent misuse or environmental contamination.
- Certificate of Registration: Once all forms and applications are approved, the applicant receives a Certificate of Registration. This certificate serves as official recognition that the facility is authorized to handle controlled substances according to regulatory requirements.
These forms and documents create a robust framework for regulated activities surrounding controlled substances. Properly completing and submitting them is essential for compliance and ensuring the integrity of the controlled substances system.
Similar forms
Form DHHS 224: This form is used for the application for registration as a controlled substance prescriber. Like Form 227, it requires detailed identification of the applicant and the type of business activity related to controlled substances.
Form DEA 106: This document is filed to report the theft or loss of controlled substances. Similar to Form 227, it addresses issues related to controlled substances, but it focuses on incidents rather than registration.
Form DEA 363: This form is for the registration of Narcotic Treatment Programs. It requires similar information regarding the applicant's qualifications and intended activity concerning controlled substances.
Form DEA 223: This is the registration application for Manufacturer or Distributor of controlled substances. Like Form 227, it requires a fee, and the applicant must indicate their type of business activity.
Form DEA 207: This document serves as a renewal application for the registration of a practitioner. It similarly verifies the identity of the applicant and their qualifications to handle controlled substances.
Form 508: This form is utilized for registering as a manufacturer, distributor, or researcher. It parallels Form 227 in that it collects information about the applicant and the substances they intend to handle.
Form 222: This is a specific order form for Schedule I and II controlled substances. Like Form 227, it requires detailed identification but is focused on ordering and not registration.
Form 3500: While primarily for adverse event reporting, it also pertains to the safety of handling substances. Like Form 227, it emphasizes the tracking and regulation of controlled substances.
Form 290: This is a document used for registering as a laboratory handling controlled substances. It requires similar compliance verification as seen in Form 227.
Dos and Don'ts
When filling out the FORM DHHS 227, it’s important to be thorough and accurate. Below is a helpful list of dos and don’ts to consider during the application process:
- Do: Carefully read the instructions provided with the form.
- Do: Ensure all entries are printed clearly or typed, avoiding any handwriting that could be misinterpreted.
- Do: Double-check your registration classification and select the correct business activity.
- Do: Verify that your drug schedules align with the activities you plan to conduct.
- Do: Retain a copy of the completed application for your records.
- Don’t: Forget to include your contact information for follow-up purposes.
- Don’t: Leave any sections blank. If a section does not apply, indicate that clearly.
- Don’t: Rely solely on automated forms or tools—personal review is crucial.
- Don’t: Submit the application without verifying the payment method and amount required.
- Don’t: Ignore the necessity of including any required documentation if you’ve answered "Yes" to specific questions.
Following these guidelines will help smooth your application process and increase the likelihood of a successful outcome. Best of luck!
Misconceptions
-
Misconception 1: The 227 form is only for manufacturers.
This form is applicable not just to manufacturers but also to distributors, researchers, and analytical laboratories. Each applicant must select their specific business activity from the provided options. Understanding your classification is crucial for compliance.
-
Misconception 2: A previous felony conviction automatically bars registration.
While a felony conviction related to controlled substances can impact your application, it does not automatically disqualify you. Each case is evaluated on its own merits, and you are often given an opportunity to explain the circumstances.
-
Misconception 3: Filling out the form is a simple matter of providing basic information.
Completing the 227 form requires careful attention to detail. Applicants must ensure that all sections are completed accurately, including specific drug schedules and applicable classifications. Failure to do so can result in delays or rejections.
-
Misconception 4: Registration guarantees approval for all controlled substances.
Registration as a manufacturer or distributor does not automatically grant permission to handle all controlled substances. Applicants must specify which drug schedules they intend to work with, and approval is contingent on that specification.
-
Misconception 5: The registration fee is the same for all applicants.
The fee structure varies by business activity. For example, manufacturers pay $600, while researchers pay only $125. Understanding these differences can help in planning and budgeting for the registration process.
-
Misconception 6: Once submitted, the application will be processed immediately.
The processing of the 227 form can take time. Applicants should allow for delays and ensure they submit their forms well in advance of any anticipated needs. Proper planning is key to avoiding interruptions in business operations.
Key takeaways
Filling out and utilizing the DHHS Form 227 is a pivotal step for entities involved with controlled substances in North Carolina. Here are some key takeaways:
- The form is specifically designed for re-registration under the North Carolina Controlled Substances Act of 1971.
- It requires accurate information, including your valid registration and DEA numbers, which must be clearly printed or typed.
- Applications should be mailed to the Department of Health and Human Services, ensuring it reaches the Controller’s Office for processing.
- Each applicant must select one business activity from a list that includes options such as manufacturer, distributor, and researcher.
- Drug schedule designations need to be thoroughly checked; applicants can select multiple schedules based on their operations.
- If any felony convictions are present relating to controlled substances, a letter explaining the circumstances must be attached to the application.
- Notably, drug code numbers must align with the selected business activities and the corresponding schedules requested.
- Maintaining a copy of the completed application is essential for your records and future reference.
- Lastly, ensure that the application is signed and dated by an authorized individual to validate the submission.
Completing the DHHS Form 227 accurately and thoroughly can facilitate the registration process and help ensure compliance with state and federal regulations.
Browse Other Templates
Qr7 Form - Using correct reporting procedures helps navigate eligibility smoothly.
Ps Form 3971 Fillable - The form serves to document essential operational metrics.
