Fill Out Your Biochemistry Basics Form
Understanding the intricate relationship between biochemistry and biology is vital for anyone delving into the life sciences. The Biochemistry Basics form serves as an essential resource for students embarking on advanced biology courses, emphasizing the pivotal concepts derived from chemistry. These include the structural properties of molecules, such as polarity and acidity, which profoundly influence biological functions. By establishing a solid grasp of how atoms combine to form essential biomolecules, learners can appreciate the underlying mechanics of life. This form is arranged into models that simplify complex concepts, providing molecular drawings and question sets that foster critical thinking. In Model 1, you will encounter illustrations of key molecules such as 1-pentanol, glucose, and unsaturated fatty acids, all represented in various drawing styles. Examining these visuals is not only instructive but also engages learners to distinguish between the representations and discern their relative accuracy. Meanwhile, Model 2 focuses on the properties of biological molecules, delineating polar and nonpolar categories while introducing terms related to functional groups. The questions prompt an exploration of the distinguishing characteristics of these molecules, enabling a deeper understanding of their biochemical roles. Addressing the intersections of acidity and basicity within biological systems, this form underscores why foundational chemistry is imperative for making sense of the molecular basis of life.
Biochemistry Basics Example
Biochemistry Basics
What concepts from chemistry are helpful in studying biology?
Why?
Typically chemistry is a prerequisite course for advanced biology courses. This is because everything in your body, everything in a plant, everything in a virus, etc. is made of atoms. The structures and properties of the molecules in an organism determine the features and properties of the organism. Which molecules are polar, which are nonpolar? Which molecules have acidic properties, which have basic prop- erties? A quick review of these concepts at the beginning of your advanced biology course will help you to understand the molecular basis for life.
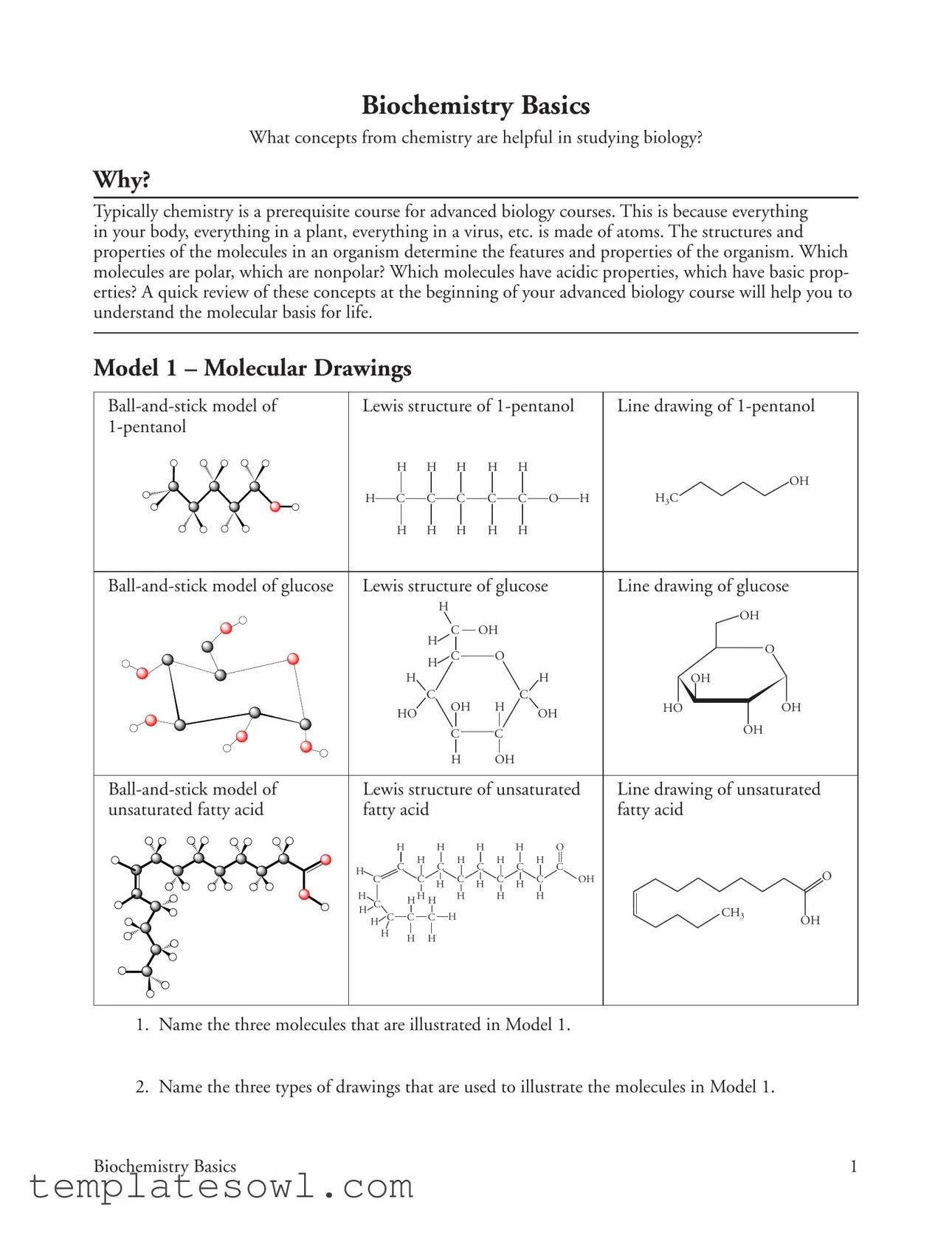
Model 1 – Molecular Drawings
Lewis structure of |
Line drawing of |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
H |
H |
|
H |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
H C |
C |
C C |
C O H |
H3C |
|
|
||||||
|
|
|
H |
H |
H |
|
H |
H |
|
|
|
|
|
Lewis structure of glucose |
|
Line drawing of glucose |
|||||||||||
|
|
|
|
H |
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
C |
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
C |
|
O |
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
H |
|
|
OH |
|
|
|
|
|
C |
|
|
|
C |
|
|
|
|
|
|
|
|
HO |
|
OH |
|
H |
|
OH |
HO |
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
C |
|
C |
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
H |
|
OH |
|
|
|
|
|
|
Lewis structure of unsaturated |
Line drawing of unsaturated |
||||||||||||
unsaturated fatty acid |
fatty acid |
|
|
|
|
|
|
fatty acid |
|
|
|||
|
|
|
H |
H |
|
H |
|
H |
|
O |
|
|
|
|
|
|
H |
C |
H |
C |
H |
C |
H |
C |
|
|
|
|
H |
|
C |
|
|
|
|
|
O |
||||
|
|
C |
|
C |
|
C |
|
C |
OH |
|
|
||
|
C |
|
H |
H |
H |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|||
|
H |
|
H H H |
H |
|
H |
|
H |
|
|
|
|
|
|
H C |
C |
C |
C |
H |
|
|
|
|
|
|
CH3 |
OH |
|
H |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
H |
H |
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
1.Name the three molecules that are illustrated in Model 1.
2.Name the three types of drawings that are used to illustrate the molecules in Model 1.
Biochemistry Basics |
1 |
3. How many bonds are typically formed by each of the following atoms:
Carbon |
Hydrogen |
Oxygen |
4.Which types of drawings in Model 1 provide more accurate images of the shape of a molecule? Justify your reasoning.
5.Refer to Model 1.
A. Symbols or atoms of what element(s) are missing from the line drawings?
B. In reading a line drawing, how do you know where atoms of these elements are in the struc- ture if they are missing from the drawing?
6.Locate the carbon and hydrogen atoms in the line drawing of isoleucine shown below and draw them in as if the drawing were a Lewis structure.
CH3 O
H3C
OH
NH2
Isoleucine
7.Isopropyl alcohol is a
8.If you were asked to write the chemical formula for one of the compounds in Model 1, which type of the drawing would be the easiest to use? Justify your reasoning.
9.What is the advantage to a scientist in using a line drawing rather than a
2 |
POGIL™ Activities for AP* Biology |
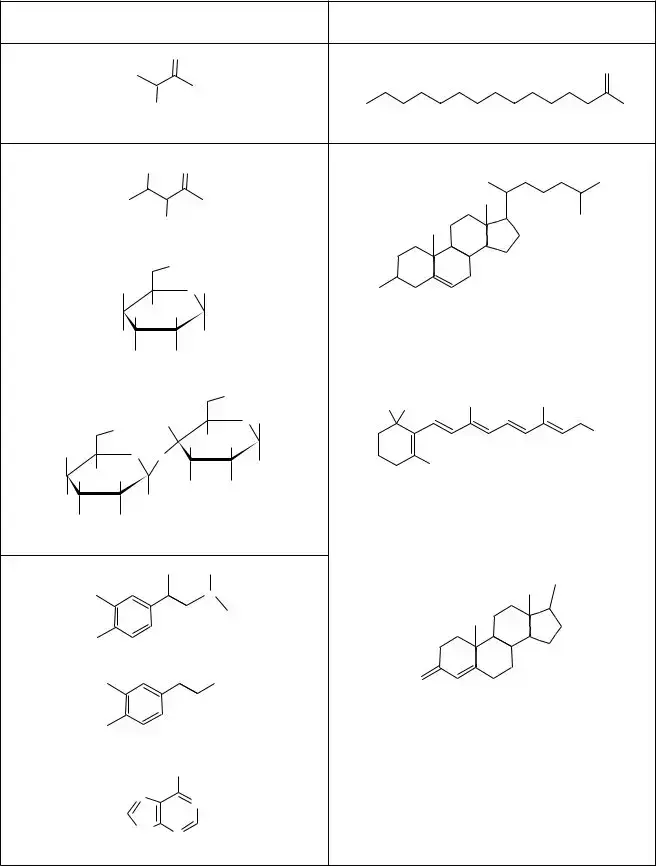
Model 2 – Properties of Biological Molecules
|
Polar Molecules |
|
|
|
Nonpolar Molecules |
||||
|
(hydrophilic) |
|
|
|
(hydrophobic) |
|
|||
Acidic |
|
|
O |
|
Acidic |
|
|
|
O |
|
H3C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
|
|
|
|
OH |
|
H3C |
|
|
|
OH |
|
|
|
|
|
|
|
|
|||
|
Lactic acid |
|
|
|
|
Fatty acid |
|
||
Neutral |
|
|
O |
|
Neutral |
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3C |
CH3 |
|
H3C |
|
|
OH |
|
|
|
CH3 |
|
|
|
NH2 |
|
|
|
|
CH3 |
CH3 |
|
Valine (amino acid) |
|
|
|
||||||
|
|
|
|
||||||
|
|
|
OH |
|
|
|
|
|
|
|
H |
|
O H |
HO |
|
|
|
|
|
|
OH H |
H |
|
|
|
|
Cholesterol |
|
|
|
HO |
|
|
OH |
|
|
|
|
|
|
H |
|
OH |
|
|
|
|
|
|
|
|
Glucose |
|
|
|
|
|
|
|
|
|
|
|
|
OH |
CH |
|
CH |
CH |
|
|
|
|
|
H C |
|
|||
|
|
|
|
|
|
|
|
|
|
|
OH |
|
H |
|
O OH |
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
|
|
OH H |
H |
|
|
|
||
|
|
|
|
|
|
|
|||
HO |
O |
O |
|
|
H |
|
|
CH |
|
OH H |
|
|
|
|
|
|
|
||
H |
|
|
|
|
|
|
|
||
|
H |
|
OH |
|
|
Vitamin A |
|
||
|
|
|
|
|
|
|
|||
H |
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
H |
OH |
|
|
|
|
|
|
|
|
|
|
Lactose |
|
|
|
|
|
|
|
Basic |
|
|
OH |
H |
|
|
|
|
OH |
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
N |
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
CH3 |
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
HO |
|
|
|
|
|
|
|
|
|
|
Adrenaline |
|
|
|
|
|
|
||
HO |
|
|
|
NH2 |
O |
|
|
||
|
|
|
|
|
|
|
|
Testosterone |
|
HO |
|
|
|
|
|
|
|
|
|
|
Dopamine |
|
|
|
|
|
|
||
|
|
|
NH2 |
|
|
|
|
|
|
|
N |
N |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||
|
NH |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adenine |
|
|
|
|
|
|
||
Biochemistry Basics |
|
|
|
|
|
|
|
3 |
|
10.Consider the polar molecules in Model 2.
A. In general, the presence of atoms of what element(s) makes a molecule polar?
B. What property do atoms of these elements have that helps make the molecules they are in polar?
C. Can nonpolar molecules also have atoms of these elements? If yes, what distinguishes a non- polar molecule from a polar molecule?
11.In chemistry there is a saying “like dissolves like,” which means things will mix with or dissolve into each other best when their polarities are similar.
A. Is water polar or nonpolar?
B. Is oil polar or nonpolar?
C. Which of the substances in Model 2 would dissolve well in water? Justify your reasoning.
D. Which of the substances in Model 2 are more likely to dissolve well in oil? Justify your reasoning.
E. Which class of substances in Model 2, polar or nonpolar, is more likely to be found in high concentrations in the bloodstream of a vertebrate? Justify your reasoning.
12.Refer to Model 2.
A. What is another term for a polar molecule?
B. What is another term for a nonpolar molecule?
C. Give the literal translation for the terms you gave in parts A and B above.
4 |
POGIL™ Activities for AP* Biology |
13.Functional groups are key groups of atoms in biological molecules. Describe the carboxyl func- tional group that both acidic molecules in Model 2 have in common.
14.Recall the definition of an acid that you learned in chemistry. Explain how the reaction below illustrates the acidic properties of lactic acid.
OO
H3C
OH
OH + H2O |
|
H3C |
|
|
|
O– |
+ H3O+ |
|
|
|
|
||||||
|
|
|
|
|
|
|||
|
||||||||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
OH
Lactic acid |
Lactate ion |
15.Describe the functional group, called an amine group, that the basic molecules in Model 2 all have in common?
16.Recall the definition of a base that you learned in chemistry. Explain how the reaction below illustrates the basic properties of adrenaline.
OH |
H |
|
HO |
N |
HO |
|
CH3 |
|
|
+ H2O |
|
|
|
|
HO |
|
HO |
|
|
Adrenaline
H
+ |
|
|
|
N |
|
CH3 |
|
|
|
||
|
|
|
|
H |
+ |
OH– |
|
|
|
|
|
17.Predict the approximate pH (pH = 7, pH > 7 or pH < 7) of fairly concentrated aqueous solu- tions of the following compounds from Model 2.
Lactic acid |
____________ |
Dopamine |
____________ |
Amino acid |
____________ |
Lactose |
____________ |
Biochemistry Basics |
5 |
18.In chemistry you learned that covalent bonds are one type of intramolecular bond. They occur between nonmetal atoms in a molecule. You may have also learned about a type of intermo- lecular bond called a hydrogen bond. Hydrogen bonds are weak attractive forces between polar molecules containing strong polar bonds such as
H O H H O H
H O
N 
 H
H
H O
O H
H H  O H
O H
A. Label at least two covalent bonds in the diagram above.
B. Label at least one hydrogen bond in the diagram above.
19.Which of the molecules in Model 2 would form hydrogen bonds with itself (that is, other mol- ecules of the same type) or with water molecules if in a solution?
6 |
POGIL™ Activities for AP* Biology |
Extension Questions
20.Although amino acids have “acid” in their name, some are acidic in water solutions, some are basic, and others are neutral. Propose an explanation for this observation based on the structures and descriptions of the amino acids below.
Neutral amino acids
OO
H3C
OH HO OH
NH2NH2
Acidic amino acid |
|
Basic amino acid |
|||||||||||
O |
|
O |
|
|
O |
||||||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
H2N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
HO |
|
|
|
|
OH |
|
|
|
OH |
||||
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
NH2 |
|
NH |
||||||||
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
21.The structure shown below is a line drawing of noncyclic AMP, an important messenger mol- ecule in molecular communication systems.
A. Draw the missing carbon and hydrogen atoms on the molecule.
|
|
|
|
NH2 |
|
|
|
|
N |
N |
|
|
O |
|
|
|
|
|
|
|
|
|
|
– |
O P |
O |
N |
N |
➾ |
|
|
|
|||
|
|
|
O |
|
|
|
|
|
|
|
OH OH
B. Write the chemical formula for a molecule of noncyclic AMP.
Biochemistry Basics |
7 |
22.The phosphate functional group in the noncyclic AMP molecule of Question 21 contains “acidic hydrogens.”
A. Explain what this phrase means.
B. Draw the noncyclic AMP molecule after it has dissolved in water.
8 |
POGIL™ Activities for AP* Biology |
Form Characteristics
| Fact Name | Fact Description |
|---|---|
| Purpose of Chemistry in Biology | Chemistry serves as a foundational discipline for understanding biological processes. Knowledge of chemical structures and reactions aids in comprehending the molecular basis of life. |
| Atomic Composition | All living organisms, including plants, animals, and viruses, are composed of atoms, which are the building blocks of matter. |
| Moleculer Properties | The features and properties of organisms are determined by the structures and properties of the molecules they contain. |
| Types of Molecules | Molecules can be classified as polar or nonpolar, based on their charge distribution, which affects their interactions with other molecules. |
| Bonds by Atoms | Typically, carbon forms four bonds, hydrogen forms one bond, and oxygen forms two bonds. |
| Visual Representation | Different types of drawings—like ball-and-stick models, Lewis structures, and line drawings—illustrate molecules in varying degrees of accuracy regarding their three-dimensional shape. |
| Missing Elements | In line drawings, certain atoms may not be explicitly shown. Their presence is often inferred from common bonding patterns. |
| Ease of Chemical Formulas | For writing chemical formulas, line drawings may be the most straightforward since they omit unnecessary details while maintaining clarity. |
| Functional Groups | Functional groups, such as carboxyl and amine groups, significantly influence the properties and reactivity of biological molecules. |
| Solubility Principles | The principle of "like dissolves like" indicates that polar molecules mix well with polar solvents, while nonpolar molecules do the same with nonpolar solvents. |
Guidelines on Utilizing Biochemistry Basics
The Biochemistry Basics form is an important tool for understanding the foundational connections between chemistry and biology. By completing this form, you will engage with key concepts that will enhance your grasp of biological structures and properties. Follow the steps below to fill out the form accurately.
- Begin by writing your name at the top of the form.
- Answer the first question by naming the three molecules illustrated in Model 1.
- Next, specify the three types of drawings used to depict these molecules in Model 1.
- List how many bonds are typically formed by each of the following atoms: Carbon, Hydrogen, and Oxygen.
- Identify which types of drawings provide more accurate images of the shape of a molecule and explain why.
- For question 5, indicate the symbols or atoms that are missing from the line drawings and describe how to identify their locations in the structure.
- Locate the carbon and hydrogen atoms in the line drawing of isoleucine, and draw them in as they would appear in a Lewis structure.
- Sketch isopropyl alcohol using all three types of drawings, as per the given description.
- Decide which drawing type would be easiest for writing a chemical formula for one of the compounds in Model 1 and explain your choice.
- Discuss the advantages of using a line drawing compared to a ball-and-stick model or Lewis structure.
- Examine the polar molecules in Model 2. Answer questions regarding the presence of certain atoms, their properties, and the distinction between polar and nonpolar molecules.
- Investigate the solubility of the substances listed in Model 2 as they relate to water and oil.
- Write down another term for a polar molecule as well as one for a nonpolar molecule, including their literal translations.
- Describe the carboxyl functional group common to the acidic molecules found in Model 2.
- Refer to the provided reaction to explain how it illustrates the acidic properties of lactic acid.
- Define the amine group that is consistently found in the basic molecules of Model 2.
- Analyze the provided reactions to explain how they demonstrate the basic properties of adrenaline.
- Finally, predict the approximate pH of various compounds from Model 2 based on your knowledge.
What You Should Know About This Form
What concepts from chemistry are helpful in studying biology and why?
Understanding basic chemistry concepts is essential for studying biology. Fundamental principles like the structure of atoms, types of molecules, and their properties can significantly enhance comprehension of biological processes. Everything in living organisms—from the cells in your body to the plants around you—is made up of atoms. These atoms form molecules, and the structures and properties of these molecules dictate how organisms function. A quick review of concepts such as polarity and acidity can provide a solid foundation for grasping the molecular basis of life as you delve deeper into biology.
What types of drawings are used to illustrate molecules, and which provides the most accurate image of a molecule's shape?
In Model 1, three types of drawings are employed: ball-and-stick models, Lewis structures, and line drawings. Ball-and-stick models give a three-dimensional view of the molecule, allowing for visual appreciation of bond angles and lengths. Lewis structures depict the arrangement of atoms and the lone pairs of electrons. Line drawings also show how atoms connect, but they simplify the representation by omitting certain atoms. Among these, the ball-and-stick model typically provides the most accurate depiction of a molecule's shape because it captures the spatial arrangement of atoms more effectively than the simplified line drawings.
How many bonds are typically formed by carbon, hydrogen, and oxygen atoms?
In general, carbon, hydrogen, and oxygen each display a characteristic bonding behavior. Carbon typically forms four bonds, allowing it to connect with various atoms and create diverse structures. Hydrogen usually forms one bond, as it has one electron to share. Oxygen, on the other hand, forms two bonds, pairing one electron from each of two other atoms. This bonding behavior is fundamental in understanding how these elements interact with one another to form larger biological molecules.
What is the advantage of using a line drawing instead of a ball-and-stick model or Lewis structure?
One significant advantage of using a line drawing is its simplicity and ease of interpretation. Line drawings reduce the clutter often associated with ball-and-stick models while clearly showing how atoms are connected. This streamlined representation can expedite the review and understanding of complex structures, particularly in advanced biological studies, where clarity and quick comprehension are crucial. Scientists often prefer line drawings for their practicality, especially when depicting large or intricate molecules.
What class of substances, polar or nonpolar, is more likely to be found in high concentrations in the bloodstream of a vertebrate?
Polar substances are more likely to be found in high concentrations in the bloodstream of vertebrates. This is primarily because blood is an aqueous solution, meaning it consists mostly of water, a polar molecule. Polar molecules dissolve well in water, allowing them to be transported effectively throughout the body. Nonpolar substances, however, tend to be hydrophobic and do not mix well with water, making it less likely for them to be present in high concentrations in the bloodstream.
How can you distinguish between polar and nonpolar molecules based on their atomic composition?
The distinction between polar and nonpolar molecules often hinges on their atomic composition. Polar molecules typically contain elements such as oxygen or nitrogen that can create an uneven distribution of electron density. This results in partial positive and negative charges within the molecule. Nonpolar molecules, in contrast, generally consist of atoms that share electrons more evenly, such as carbon and hydrogen. While both types can contain these elements, their overall shapes and the arrangements of their bonds determine whether the molecule is polar or nonpolar.
Common mistakes
Completing the Biochemistry Basics form requires careful attention to detail. One of the most common mistakes is answering questions without thoroughly reading the instructions. Each question has specific requirements, and overlooking these can lead to incomplete or irrelevant answers. For instance, if a question asks for justification, failing to provide a rationale can result in a lower score.
Another frequent error arises from misunderstanding the visual representations in Model 1. People might name the correct molecules but fail to correlate them with the correct types of drawings. Be sure to clearly relate each molecule to its respective representation. This confusion can mislead the reviewer and undermine your efforts.
The third mistake involves neglecting fundamental chemistry concepts. Some individuals might rush through questions about atomic bonds. Each atom can form a different number of bonds, and without this knowledge, answers can be inaccurate. Strong foundational understanding is crucial for success.
Additionally, many people struggle with identifying atoms missing from line drawings. A common oversight is not recognizing what these omissions imply about the structure of the molecule. Failing to grasp this aspect limits the ability to read and interpret complex structures accurately.
Another error often seen is inconsistent drawing techniques. When asked to represent isopropyl alcohol or any other molecule, individuals may switch between various drawing styles without following the guidelines. Maintaining consistency across all three types of drawings is vital for clear communication and understanding.
Moreover, responses to the question regarding the advantages of line drawings can be vague. Applicants might provide generic statements that do not adequately address the question. Specific advantages, such as clarity in representing molecular connections, should be articulated.
Lastly, people may overlook the need for context in their answers. For example, while responding to questions about polar and nonpolar molecules, it’s important to briefly explain the significance of these terms. Providing context will enhance the overall quality of the response and improve comprehension for the reviewer.
Documents used along the form
When diving into the world of biochemistry, it’s important to have a variety of forms and documents at your fingertips. These documents not only help with comprehension but also enhance your learning experience in a structured way. Below is a list of forms often used alongside the Biochemistry Basics form, providing essential support for anyone engaging in this fascinating subject.
- Laboratory Safety Guidelines: This document outlines the essential safety practices to follow while conducting experiments in a lab setting. It includes information on proper handling of chemicals, use of personal protective equipment, and emergency procedures.
- Experiment Protocols: Detailed step-by-step instructions for conducting specific experiments. These protocols ensure consistency and accuracy in experimental procedures, making it easier to replicate results.
- Data Collection Sheet: A handy form for recording observations, measurements, and other vital data during laboratory experiments. This helps in organizing information systematically for analysis later on.
- Chemical Inventory Form: This document lists all chemicals available in the lab, including their concentrations, quantities, and storage locations. Keeping an up-to-date inventory ensures that necessary materials are always accessible.
- Biochemistry Lab Report Template: A structured outline for compiling lab reports, including sections for introduction, methods, results, and discussion. This template guides students in presenting their findings clearly and logically.
- Reference Literature Checklist: This document helps students and researchers track relevant publications, articles, and textbooks they should consult while studying or conducting research in biochemistry.
- Study Plan Outline: A personalized roadmap that breaks down topics to cover in preparation for exams or projects. This keeps learners focused and organized as they navigate through complex material.
- Glossary of Biochemical Terms: A reference list of common terms and definitions in biochemistry. This is perfect for quick look-ups and helps bolster understanding of the subject matter.
- Ethics in Biochemistry Research Guidelines: A set of ethical standards and considerations for conducting research in biochemistry. This document emphasizes the importance of integrity and respectful treatment of biological materials.
Having these documents by your side while working with the Biochemistry Basics form can make a significant difference in your educational journey. Each document serves a unique purpose, enhancing your understanding of biochemistry and helping you to engage confidently with the material.
Similar forms
The Biochemistry Basics form has similarities with several other educational documents. Each of these documents serves to provide foundational knowledge in the study of biochemistry and molecular biology.
- Laboratory Manual for Biochemistry: This manual often includes practical exercises that mirror the molecular illustrations found in the Biochemistry Basics form. It emphasizes the importance of visualizing molecular structures through diagrams and models, making complex concepts accessible to students.
- Conceptual Framework in Molecular Biology: This document outlines key concepts, similar to those in the Biochemistry Basics form. It discusses the relationship between molecular structure and biological function, providing a theoretical background that complements the practical illustrations in the Biochemistry Basics.
- Guide to Organic Chemistry for Biologists: This guide helps students understand the principles of organic chemistry that are essential for studying biological molecules. It highlights the significance of molecular properties, such as polarity and acidity, which are also discussed in the Biochemistry Basics form.
- Textbook on Biochemical Principles: A comprehensive resource that covers fundamental biochemical concepts that parallel those presented in the Biochemistry Basics form. It examines how chemical properties influence biological function, thereby reinforcing the connection between chemistry and biology.
Dos and Don'ts
When filling out the Biochemistry Basics form, it’s essential to follow certain guidelines to ensure accuracy and clarity. Here are ten things to consider:
- Do read the instructions carefully before starting.
- Do use clear and legible handwriting if completing by hand.
- Do label diagrams with appropriate details.
- Do double-check your answers for completeness.
- Do focus on using the correct scientific terminology where applicable.
- Don’t leave any sections blank unless instructed otherwise.
- Don’t rush through the questions; take your time to think.
- Don’t use abbreviations that may be unclear or undefined.
- Don’t ignore the molecular structures; they are crucial for understanding.
- Don’t submit the form without reviewing for spelling and grammatical errors.
Misconceptions
Misconceptions regarding the Biochemistry Basics form can lead to confusion and misinterpretation of essential concepts. Below are seven common misunderstandings, each with an explanation to clarify these points.
- 1. Chemistry knowledge is not necessary for biology. Many believe that studying biology does not require a strong foundation in chemistry. However, chemistry is essential, as the principles of atom structure and molecular interactions underpin all biological processes.
- 2. All molecules in biology are either polar or nonpolar. While many molecules can be classified as polar or nonpolar, biochemistry encompasses a broader range. Some molecules may have both polar and nonpolar regions, affecting their behavior and functionality in biological systems.
- 3. Molecules can exist independently of each other. Some may think that biological molecules function in isolation. In reality, molecules often interact in complex ways, forming structures like membranes and proteins vital for life.
- 4. Molecular drawings are only representations, not critical tools. The assumption that molecular drawings are merely illustrative can be misleading. Accurate representations are crucial for understanding molecular shape and function, guiding scientific research and education.
- 5. All biological molecules react similarly. It’s a common misconception that all molecules behave the same way chemically. Each molecule has unique structural properties, leading to specific interactions and reactions based on its functional groups.
- 6. Understanding acids and bases is not relevant in biology. This belief overlooks the importance of pH and the role of acidic and basic molecules in biological reactions. The behavior of many biochemical processes is heavily influenced by the acidic or basic nature of the compounds involved.
- 7. The only functional groups in biological molecules are carboxyl and amine. While carboxyl and amine groups are significant, they are just two examples. Numerous functional groups exist, each contributing distinct characteristics and reactivity to the compounds they are part of.
Being aware of these misconceptions can enhance comprehension and foster a deeper understanding of biochemistry as it relates to biology.
Key takeaways
Understanding the Biochemistry Basics Form is an essential step for those engaged in advanced biology studies. The insights gained from this form can enhance your comprehension of how molecules interact in biological systems. Here are some key takeaways regarding the use and benefits of the Biochemistry Basics form:
- The form serves as a foundational tool that connects concepts from chemistry to biological processes. Grasping the molecular structures and properties is essential for understanding life at a cellular level.
- There are various methods employed to visualize molecules, including Lewis structures, ball-and-stick models, and line drawings. Understanding the strengths and limitations of each type of drawing aids in clearer communication of molecular structures.
- This form emphasizes the importance of polar and nonpolar molecules and their interactions. Recognizing which substances mix well together, based on polarity, is crucial in predicting biological behavior, such as solubility and compatibility.
- The questions posed in the form guide users to apply knowledge practically and critically. They encourage exploration of functional groups, acidic and basic properties, and the implications of these characteristics in biological systems.
Browse Other Templates
How to Close a Delaware Corporation - Verify corporation name accuracy on the Delaware Division of Corporations website.
Mazzios Jobs - Indicate whether you can lift heavy items, if necessary.