Fill Out Your Catamaran Prior Auth Form
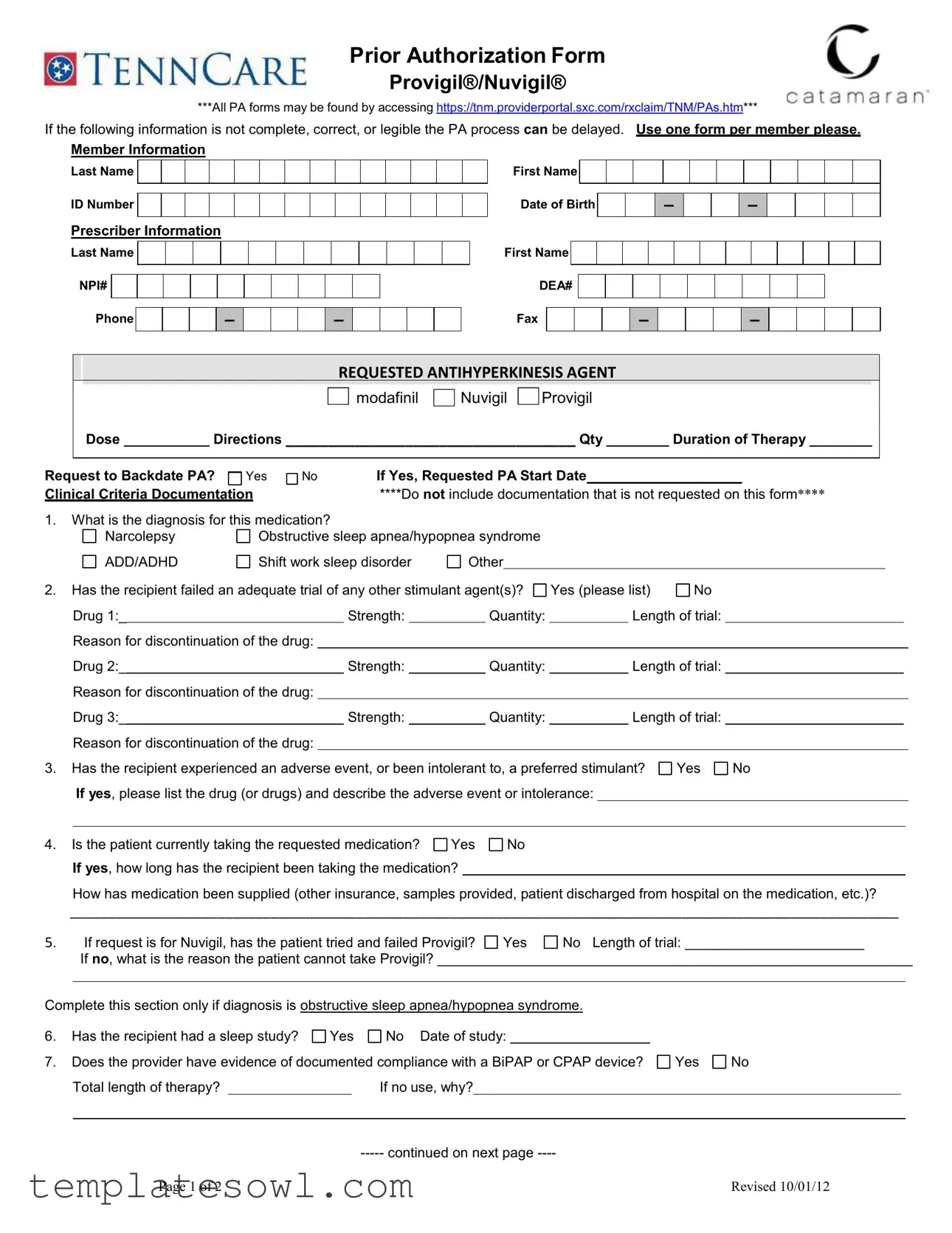
The Catamaran Prior Authorization form is crucial for healthcare providers seeking approval for medications like Provigil® and Nuvigil®. This comprehensive document ensures that essential patient and prescriber information is accurately captured to facilitate a smooth authorization process. The form requires specific details, including the patient's last name, ID number, date of birth, and the prescriber's contact information, including their NPI and DEA numbers. It also mandates clear documentation of the requested antihyperkinesis agent, dosage, duration of therapy, and clinical criteria that justify the need for the medication. Questions regarding the patient's diagnosis—such as narcolepsy or obstructive sleep apnea—are pivotal, as is evidence of prior medication trials and experiences with preferred stimulants. Additional sections address sleep studies and compliance with devices like BiPAP or CPAP. Importantly, the prescriber’s signature verifies the accuracy of the submitted information, which is necessary for approval. Properly completing each section will help prevent delays in the authorization process, allowing patients to receive the treatments they need in a timely manner.
Catamaran Prior Auth Example
Prior Authorization Form
Provigil®/Nuvigil®
***All PA forms may be found by accessing https://tnm.providerportal.sxc.com/rxclaim/TNM/PAs.htm***
If the following information is not complete, correct, or legible the PA process can be delayed. Use one form per member please.
Member Information
Last Name
ID Number
Prescriber Information
First Name
Date of Birth
–
– 
Last Name
NPI#
Phone
 –
– 
 –
– 
First Name
DEA#
Fax
–
 –
– 

REQUESTED ANTIHYPERKINESIS AGENT

 modafinil
modafinil 
 Nuvigil
Nuvigil 
 Provigil
Provigil
Dose ___________ Directions __________________________________ Qty ________ Duration of Therapy ________
Request to Backdate PA? |
Yes |
No |
If Yes, Requested PA Start Date |
|
|
Clinical Criteria Documentation |
|
****Do not include documentation that is not requested on this form**** |
|||
1.What is the diagnosis for this medication?
|
|
Narcolepsy |
Obstructive sleep apnea/hypopnea syndrome |
|
|
|
|
|
|
|
|
|||||||
|
|
ADD/ADHD |
Shift work sleep disorder |
Other |
|
|
|
|
|
|
|
|
|
|||||
2. |
Has the recipient failed an adequate trial of any other stimulant agent(s)? |
Yes (please list) |
No |
|
|
|
|
|||||||||||
|
|
Drug 1:_ |
|
|
|
Strength: |
|
|
Quantity: |
|
|
Length of trial: |
|
|
|
|
||
|
|
Reason for discontinuation of the drug: |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
Drug 2:_ |
|
|
|
Strength: |
|
|
Quantity: |
|
|
Length of trial: |
|
|
|
|
||
|
|
Reason for discontinuation of the drug: |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
Drug 3:_ |
|
|
|
Strength: |
|
|
Quantity: |
|
|
Length of trial: |
|
|
|
|
||
|
|
Reason for discontinuation of the drug: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
3. |
Has the recipient experienced an adverse event, or been intolerant to, a preferred stimulant? |
Yes |
No |
|
||||||||||||||
|
|
If yes, please list the drug (or drugs) and describe the adverse event or intolerance: |
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. |
Is the patient currently taking the requested medication? |
Yes |
|
No |
|
|
|
|
|
|
|
|
||||||
If yes, how long has the recipient been taking the medication?
How has medication been supplied (other insurance, samples provided, patient discharged from hospital on the medication, etc.)?
___________________________________________________________________________________________________________
5. |
|
If request is for Nuvigil, has the patient tried and failed Provigil? |
Yes |
No Length of trial: _______________________ |
||||||||||
|
|
If no, what is the reason the patient cannot take Provigil? _____________________________________________________________ |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|||||
Complete this section only if diagnosis is obstructive sleep apnea/hypopnea syndrome. |
|
|
|
|
||||||||||
6. |
Has the recipient had a sleep study? |
Yes |
No Date of study: |
|
|
|
|
|
|
|||||
7. |
Does the provider have evidence of documented compliance with a BiPAP or CPAP device? |
Yes |
No |
|||||||||||
|
|
Total length of therapy? |
|
|
If no use, why? |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Page 1 of 2 |
|
|
|
|
|
|
|
|
Revised 10/01/12 |
|||

TennCare Prior Authorization Form: Provigil®/Nuvigil™
– Page 2 –
Patient Name: |
|
DOB |
||
|
|
|
|
|
Complete this section only if diagnosis is shift work sleep disorder.
8. Does the patient work a minimum of 6 hours work between the hours of 10 pm and 8 am?
Yes
No
Please note any other information pertinent to this PA request:
Prescriber Signature (REQUIRED): |
|
Date: |
(By signature, the physician confirms the above information is accurate and verifiable by patient records.)
Fax This Form to:
Mail requests to: Catamaran PA Department, P.O. Box 3214, Lisle IL
Telephone
Catamaran will provide a response within 24 hours day upon receipt.
This facsimile transmission contains legally privileged and confidential information intended for the parties identified below.
If you have received this transmission in error, please immediately notify us by telephone and return the original message to P.O. Box 3214; Lisle, IL
Distribution, reproduction or any other use of this transmission by any party other than the intended recipient is strictly prohibited.
Page 2 of 2 |
Revised 10/1/2012 |
Form Characteristics
| Fact Name | Description |
|---|---|
| Purpose | This form is used to request prior authorization for the medications Provigil® and Nuvigil®, which are prescribed for conditions like narcolepsy and sleep disorders. |
| Completeness Requirement | To avoid delays in the authorization process, all fields must be filled out completely, correctly, and legibly. |
| Submission Guidelines | Requests must be faxed to 866-434-5523 or mailed to Catamaran PA Department at P.O. Box 3214, Lisle, IL 60532-8214. |
| Response Time | Catamaran promises to provide a response within 24 hours after receiving the request. |
Guidelines on Utilizing Catamaran Prior Auth
Completing the Catamaran Prior Authorization form requires careful attention to detail. It is crucial to accurately fill in all fields to avoid delays in the processing of the request. Following the steps outlined will help ensure that your submission meets the necessary requirements for approval.
- Access the form by visiting this link.
- Fill out the Member Information section:
- Last Name
- First Name
- ID Number
- Date of Birth - input in the format MM/DD/YYYY
- Complete the Prescriber Information section:
- Prescriber's Last Name
- Prescriber's First Name
- NPI Number
- Phone Number
- DEA Number
- Fax Number
- Fill in the Requested Antihyperkinesis Agent details:
- Select the medication: modafinil, Nuvigil, or Provigil
- Specify the dose
- Write directions for use
- Indicate the quantity
- State the duration of therapy
- Indicate if you request to backdate the PA and, if so, the requested start date
- Answer the Clinical Criteria Documentation questions:
- State the diagnosis for the medication from the options given.
- Specify if the recipient has failed trials of other stimulant agents. Provide details if applicable.
- Indicate if the recipient experienced any adverse events from a preferred stimulant.
- Confirm if the patient is currently taking the requested medication and provide details.
- If requesting Nuvigil, disclose if the patient has tried and failed Provigil.
- For diagnoses related to obstructive sleep apnea, reference if a sleep study was conducted and detail compliance with any prescribed device.
- For shift work sleep disorder, confirm if the patient meets the work hour requirements.
- Add any additional pertinent information to the PA request.
- Have the prescriber sign and date the form to confirm the accuracy of the provided information.
- Fax the completed form to 866-434-5523 or mail it to Catamaran PA Department, P.O. Box 3214, Lisle, IL 60532-8214.
What You Should Know About This Form
1. What is the purpose of the Catamaran Prior Authorization form?
The Catamaran Prior Authorization form is used by healthcare providers to request approval for specific medications, such as Provigil® and Nuvigil®. This authorization ensures that the prescribed medication is medically necessary and meets the clinical criteria set by the insurance provider. Completing the form accurately helps to expedite the approval process.
2. Where can I find the Prior Authorization forms?
All Prior Authorization forms are available online. They can be accessed through the following link: Catamaran Provider Portal. It is crucial to use the correct form for each medication to avoid delays.
3. What information is required to be filled out on the form?
The form requires essential information, including the member's name, ID number, and date of birth, as well as the prescriber's name, NPI number, and DEA number. Additionally, you must specify the requested medication, dosage, duration of therapy, and any clinical criteria documentation needed to support the request.
4. What happens if I submit an incomplete or illegible form?
Submitting an incomplete or illegible form may delay the Prior Authorization process. It is vital to ensure that all sections of the form are fully completed and that the handwriting is legible. Double-checking the information before submission can prevent unnecessary delays in receiving the authorization.
5. How long does it take to receive a response after submitting the form?
Catamaran aims to provide a response within 24 hours of receiving the completed Prior Authorization form. Prompt submission of the correct information can facilitate timely processing.
6. Can the Prior Authorization request be backdated?
Yes, there is an option on the form to request backdating the Prior Authorization, which would allow the authorization to take effect from a specified earlier date. However, the reason for backdating must be justified clearly along with the request.
7. Is it necessary for the prescriber to sign the form?
Yes, the prescriber must sign the form. By signing, the physician confirms that the information provided is accurate and can be verified through patient records. This signature is a crucial part of the authorization process.
8. What should I include as clinical criteria documentation?
It is important to only include the documentation specifically requested on the form. This may involve details about the diagnosis, previous treatments, and any trials of other medications. Including extraneous information can lead to complications in the approval process.
9. What if the required diagnosis is obstructive sleep apnea?
If the diagnosis is obstructive sleep apnea or hypopnea syndrome, the form requires additional questions, such as whether a sleep study has been conducted and evidence of compliance with any prescribed devices like BiPAP or CPAP. This information is critical for assessing the medical necessity of the requested medication.
10. How do I submit the form after completing it?
Once the form is completed, it can be submitted via fax or mail. The fax number for submissions is 866-434-5523, and mailed requests should be sent to Catamaran PA Department, P.O. Box 3214, Lisle, IL 60532-8214. Ensure that the form is sent to the correct location for prompt processing.
Common mistakes
Filling out the Catamaran Prior Authorization form requires careful attention to detail. One common mistake is not providing complete member information. For example, omitting the last name or ID number can lead to delays in processing. Each form should have all relevant details about the member to ensure swift approval.
A second mistake involves inaccurate or incomplete prescriber information. It's essential to include the prescriber’s first and last name, NPI number, and contact details like phone and fax numbers. Missing any of these elements can prevent the review from taking place.
Many individuals overlook the importance of precise dosing and therapy duration. Filling out the dose, directions, quantity, and duration of therapy accurately is crucial. If this section is left blank or filled inaccurately, it could lead to unnecessary back-and-forth communication, delaying the authorization process.
The fourth mistake is failing to answer clinical criteria questions completely. When asked about the patient’s diagnosis or previous medication trials, it's vital to provide detailed, truthful responses. Incomplete answers often result in inquiries that could have been avoided.
In addition, some applicants neglect to document existing medication regimens adequately. If a patient is already taking the requested medication, including how long they have been on it is critical. This history can be significant in approving the new request.
Details regarding prior stimulant trials can also be mishandled. If a recipient has tried other medications, they must specify strength, quantity, and reasons for discontinuation. Inaccuracies or omissions here can lead to rejection of the prior authorization.
When the request pertains to Nuvigil, it's essential to clarify whether the patient has attempted and failed Provigil. Sometimes, applicants forget to document this trial. This essential information can heavily influence the outcome of the request.
The penultimate error involves an incomplete section regarding sleep studies. If diagnosing obstructive sleep apnea, details such as the date of the study must be provided. Failure to include this information may raise questions about necessity.
Finally, prescribers must remember that their signature is required on the form. Without it, the authorization cannot proceed. The signature confirms that the information provided is accurate and can be verified by patient records.
Documents used along the form
When seeking a prior authorization for medications such as Provigil® or Nuvigil®, several other forms and documents can assist in ensuring a smooth approval process. The following list outlines some of these essential documents, highlighting their purpose and importance in the overall procedure.
- Clinical Documentation: This includes medical records and notes from the healthcare provider that support the need for the requested medication. It serves as evidence of the patient's diagnosis and any relevant treatments they have previously received.
- Medication History Report: This document outlines the patient’s past and current medications, providing insights into what previous treatments have been tried. It is vital for showing medication efficacy and any adverse reactions the patient may have experienced.
- Insurance Policy Information: A summary of the patient's current insurance coverage can clarify what medications are covered. This will help ensure the prior authorization request aligns with the insurer's policies.
- Statement of Medical Necessity: A detailed letter from the prescribing physician explaining why the requested medication is necessary for the patient’s treatment. This statement often addresses alternative treatments that may not be appropriate for the patient.
- Sleep Study Report: For patients with diagnoses related to sleep disorders, this report provides critical data from conducted sleep studies. It may include details such as Apnea-Hypopnea Index (AHI) and any prescribed therapies related to sleep issues.
- Pharmacy Records: Documentation from the pharmacy indicating prior fills of the requested medication can help establish a history of compliance and the need for continued therapy, especially if the medication is frequently prescribed or changed.
- Patient Consent Form: This form may be necessary to confirm that the patient is informed about the treatment and consents to the sharing of their medical information for authorization purposes.
These interconnected documents contribute significantly to the prior authorization process. Providing complete, accurate information aids in expediting approvals, thus ensuring that patients receive their necessary medications without unnecessary delays.
Similar forms
- Medicare Prior Authorization Form: Similar to the Catamaran Prior Auth form, this document requires complete patient and provider information. Both forms aim to ensure that necessary medical evidence supports the request for specific medication, streamlining approval processes.
- Blue Cross Blue Shield Prior Authorization Request: This form also gathers detailed information regarding diagnosis and treatment history. Both documents verify that patients have explored alternative treatments before requesting specific medications.
- Aetna Precertification Request: Like the Catamaran form, it seeks thorough documentation of medical necessity. Providers must outline the patient's treatment plan and any previous medications tried.
- UnitedHealthcare Authorization Form: This form serves a similar function as the Catamaran document by requiring a diagnosis and medication-specific information. Both focus on ensuring that appropriate treatment guidelines are followed.
- Cigna Prior Authorization Request: This document shares the requirement for detailed clinical criteria. Both forms aim to confirm that patients meet predefined criteria before medication approval.
- Humana Prior Authorization Form: This form, like the Catamaran version, asks for details on prior treatments and any failures associated with them to substantiate the request for medication.
- Medicaid Prior Authorization Form: This form requires similar patient identification and clinical documentation. Both forms are designed to assess the medical necessity of requested treatments based on established criteria.
- Tricare Prior Authorization Form: This document follows the same principles as the Catamaran form, focusing on the verification of medical necessity and appropriate justification for medication use.
Dos and Don'ts
When filling out the Catamaran Prior Auth form, consider the following:
- Do use one form per member to avoid confusion.
- Don't submit incomplete, incorrect, or illegible information as it may delay the process.
- Do clearly fill in all required fields, including member and prescriber information.
- Don't include any documentation that is not requested on the form.
- Do specify the diagnosis for the medication being requested.
- Don't forget to list any previous stimulant medications and reasons for discontinuation.
- Do provide details about the patient's current medication use, if applicable.
- Don't overlook the signature section; it is required for submission.
- Do fax or mail the form to the correct address for prompt processing.
Misconceptions
Prior Authorization (PA) forms can often lead to misunderstandings, particularly the Catamaran Prior Auth form for medications like Provigil and Nuvigil. Here are some common misconceptions.
-
Misconception 1: The PA form is optional for all requests.
In reality, submitting the Catamaran PA form is mandatory for certain medications. Without it, approval for coverage may be delayed or denied entirely. This form is vital for compliance with the insurance provider's requirements. -
Misconception 2: All information can be submitted later if forgotten.
Once a PA request is sent in, additional information cannot be added later. If the form lacks crucial details, the approval process will be stalled. Completing all sections fully and accurately on the initial submission is essential. -
Misconception 3: Only the physician needs to fill out the form.
It often takes collaboration between the prescriber and the patient to gather the necessary information. Patients may have insights or documentation that support the need for the medication, which is vital in the approval process. -
Misconception 4: You can fax the form to any number.
There is a specific fax number provided for submissions, which is 866-434-5523. Sending the form to any other number could result in it not being processed, causing further delays.
Understanding these points helps ensure a smoother experience when filling out the Catamaran Prior Auth form. It encourages accuracy and collaboration, ultimately leading to better medical care continuity.
Key takeaways
Filling out the Catamaran Prior Authorization form requires attention to detail and accuracy. Here are some key takeaways to keep in mind:
- Use One Form Per Member: Each member must have their own form to prevent processing delays.
- Complete Information is Crucial: Ensure that all sections are filled out completely, legibly, and accurately to avoid any interruptions in the prior authorization process.
- Provide Diagnosis Information: Clearly indicate the diagnosis for which the medication is being requested. Selecting the correct condition is essential.
- Medication History Matters: Document any prior stimulant medications the patient has tried, including dosages, duration, and reasons for discontinuation. This information can significantly impact the approval process.
- Document Adverse Events: If the patient has experienced adverse reactions to preferred treatments, this needs to be detailed on the form.
- Supplier Information Required: Include how the patient is currently obtaining the medication, even if they had prior insurance coverage or received samples.
- Timely Submission is Key: After completing the form, fax it to 866-434-5523 or mail it to the provided address. A response will come within 24 hours of receipt, so prompt submission is advised.
Overall, careful attention to the details requested in the form can lead to a smoother authorization process for the necessary medication.
Browse Other Templates
Behind the Wheel Practice Log - Practice in varying environments improves overall driving competence.
60th Wedding Anniversary Card From King - A century of memories to be celebrated and cherished.