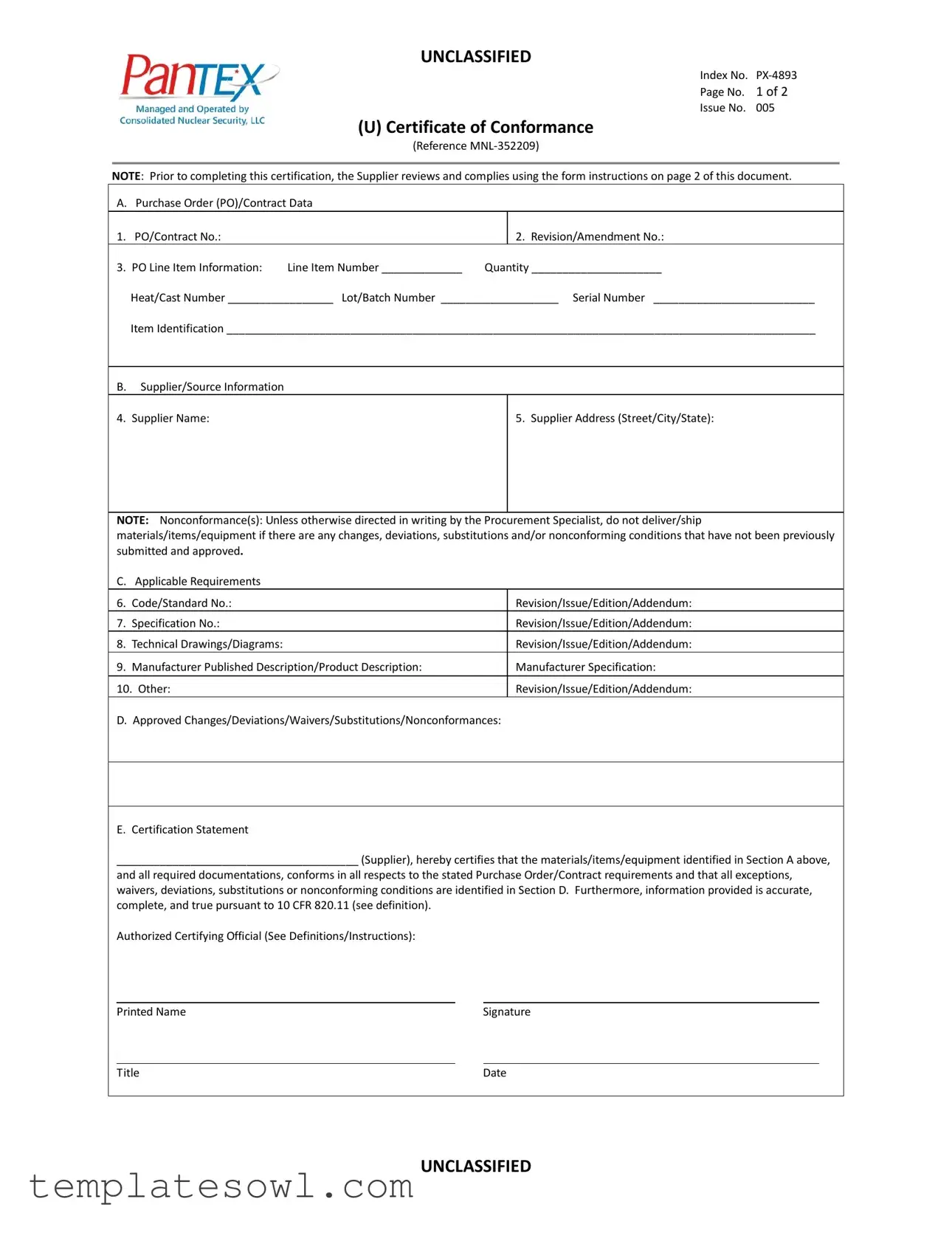
Fill Out Your Certificate Of Conformance Form
The Certificate of Conformance (C of C) form plays a crucial role in ensuring that supplied materials, items, or equipment meet the specific standards outlined in a purchase order or contract. This important document covers various sections that detail essential information, starting with the Purchase Order (PO) and contract data, where suppliers list identification numbers and quantities related to each item. Venturing further, it provides space for suppliers to input their company details to establish clear communication between parties. The form also emphasizes the need to comply with applicable requirements, including codes, standards, and specifications, making it clear that accuracy is key. Additionally, suppliers must identify any approved changes or deviations, ensuring transparency and adherence to agreed-upon expectations. The certification statement lies at the heart of the form, where an authorized official vouches for the integrity of the information provided, confirming that it corresponds to all specified requirements. Prior to completing this certification, suppliers must thoroughly review the instructions to ensure compliance with all conditions, particularly regarding nonconformances, which may impact the delivery of goods. In short, the C of C form is not just a simple document but a vital assurance that fosters quality and accountability in supplier relationships.
Certificate Of Conformance Example
UNCLASSIFIED
Index No.
Page No. 1 of 2
Issue No. 005
(U) Certificate of Conformance
(Reference
NOTE: Prior to completing this certification, the Supplier reviews and complies using the form instructions on page 2 of this document.
A. Purchase Order (PO)/Contract Data
1. |
PO/Contract No.: |
|
|
2. Revision/Amendment No.: |
3. |
PO Line Item Information: |
Line Item Number _____________ |
Quantity _____________________ |
|
|
Heat/Cast Number _________________ Lot/Batch Number ___________________ Serial Number __________________________ |
|||
|
Item Identification _______________________________________________________________________________________________ |
|||
|
|
|
|
|
B. |
Supplier/Source Information |
|
|
|
4. |
Supplier Name: |
|
|
5. Supplier Address (Street/City/State): |
NOTE: Nonconformance(s): Unless otherwise directed in writing by the Procurement Specialist, do not deliver/ship materials/items/equipment if there are any changes, deviations, substitutions and/or nonconforming conditions that have not been previously submitted and approved.
C. |
Applicable Requirements |
|
6. |
Code/Standard No.: |
Revision/Issue/Edition/Addendum: |
7. |
Specification No.: |
Revision/Issue/Edition/Addendum: |
8. |
Technical Drawings/Diagrams: |
Revision/Issue/Edition/Addendum: |
9. |
Manufacturer Published Description/Product Description: |
Manufacturer Specification: |
10. Other: |
Revision/Issue/Edition/Addendum: |
|
D. Approved Changes/Deviations/Waivers/Substitutions/Nonconformances:
E. Certification Statement
_______________________________________ (Supplier), hereby certifies that the materials/items/equipment identified in Section A above,
and all required documentations, conforms in all respects to the stated Purchase Order/Contract requirements and that all exceptions, waivers, deviations, substitutions or nonconforming conditions are identified in Section D. Furthermore, information provided is accurate, complete, and true pursuant to 10 CFR 820.11 (see definition).
Authorized Certifying Official (See Definitions/Instructions):
Printed Name |
|
Signature |
|
|
|
Title |
|
Date |
UNCLASSIFIED

UNCLASSIFIED
Index No.
Page No. 2 of 2
Issue No. 005
(U) Certificate of Conformance
(Reference
Instructions
Prepare a Certificate of Conformance (C of C) addressing each PO line item, Contract Deliverable, or each partial shipment. Unless otherwise specified, the C of C accompanies each shipment. All applicable form entries are completed.
A Supplier
Definitions
Authorized Certifying Official - The certification is attested to by an authorized representative of the supplier; and the certification system, including the procedures for completing, reviewing, and approving the certificate are described in the Company’s administrative control system or Quality Assurance program.
Certification - The act of determining, verifying, and attesting in writing to the qualifications of personnel, processed, procedures, or items in accordance with specified requirements.
Certificate of Conformance - A document signed or otherwise authenticated by an authorized individual certifying the degree to which items or services meet specified requirements.
10 CFR 820.11 - Procedural Rules for DOE Nuclear Activities, Subpart “A”, Information requirements. The regulation states: Any information pertaining to a nuclear activity provided to DOE by any person or maintained by any person for inspection by DOE are complete and accurate in all material respects.
No person involved in a DOE nuclear activity conceals or destroys any information concerning a violation of a DOE Nuclear Safety Requirement, a Nuclear Statute, or the Act.
Section A, Purchase Order (PO)/Contract Data
Entry 1 Enter the complete CNS Pantex PO or Contract Number.
Entry 2 Enter PO/Contract Revision or Amendment Number, (if applicable).
Entry 3 Enter as applicable, the PO Line Item Number (i.e., 1, 2, 3), quantity, heat/cast number, lot/batch number, serial number, and item identification.
Section B, Supplier/Source Information
Entry 4 Enter the legal Supplier company name, as stated on the PO or Contract.
Entry 5 Enter the Supplier business address, as stated on the PO or Contract.
Section C, Applicable Requirements
Entry 6 Enter the applicable design code/standard number and applicable revision, issue, edition, or addendum.
Entry 7 Enter the applicable specification number and applicable revision, issue, edition, or addendum.
Entry 8 Enter the applicable technical drawing/diagram and applicable revision, issue, edition, or addendum.
Entry 9 Mark the applicable box manufacturer published description/product description or manufacturers specification.
Entry 10 Enter other applicable requirements documents and applicable revision, issue, edition, or addendum.
Section D, Approved Changes/Deviations/Waivers/Substitutions/Nonconformances
Enter any approved changes. Reference change documentation control numbers as applicable. (Attach additional pages if necessary).
Section E, Certification Statement (see definitions)
Enter the Company name (or commonly used acronym).
Print or type the authorized company certifying officials name, title, and date.
Sign or otherwise authenticate by company certifying official.
Transmittal:
CNS Pantex, LLC
Fax:
Attn: SUPPLIER QUALITY
Or
Place a copy with the shipment.
UNCLASSIFIED
Form Characteristics
| Fact Name | Fact Details |
|---|---|
| Purpose | The Certificate of Conformance certifies that materials or items meet specified contract requirements. |
| Sections | The form contains five main sections: Purchase Order Data, Supplier Information, Applicable Requirements, Approved Changes, and Certification Statement. |
| Supplier Review | Suppliers must review and comply with form instructions before completing the certificate. |
| Authorized Official | An authorized certifying official must sign the certification statement, verifying compliance. |
| Regulatory Compliance | The form complies with 10 CFR 820.11, ensuring accurate information related to nuclear activities. |
| Delivery Restrictions | Nonconformance findings must be reported before delivery; shipments cannot include unapproved changes. |
| Form Variants | Variants of the form may exist based on specific contracts or state regulations. |
| Governing Law | Each state may have specific regulations governing the Certificate of Conformance, requiring adherence to local laws. |
Guidelines on Utilizing Certificate Of Conformance
Completing the Certificate of Conformance form is essential for ensuring that all materials supplied meet the necessary requirements. Following these steps will help you accurately fill out the form and facilitate a smooth approval process.
- Section A: Purchase Order (PO)/Contract Data
1. Enter the complete Purchase Order or Contract Number.
2. If applicable, enter the Revision or Amendment Number.
3. Provide the necessary details for the applicable line item: Line Item Number, Quantity, Heat/Cast Number, Lot/Batch Number, Serial Number, and Item Identification. - Section B: Supplier/Source Information
4. Input the legal Supplier company name as specified in the PO or Contract.
5. Fill out the Supplier business address, including Street, City, and State, as per the PO or Contract. - Section C: Applicable Requirements
6. Enter the design code/standard number along with the relevant revision, issue, edition, or addendum.
7. Specify the applicable specification number and its corresponding revision, issue, edition, or addendum.
8. Include the technical drawing/diagram information with the respective revision, issue, edition, or addendum.
9. Mark the appropriate box for either the manufacturer published description/product description or the manufacturer specification.
10. List any other required documents and their applicable revisions, issues, editions, or addendums. - Section D: Approved Changes/Deviations/Waivers/Substitutions/Nonconformances
Enter any approved changes and reference any associated documentation control numbers. If necessary, attach additional pages for more details. - Section E: Certification Statement
Enter the Company name or commonly used acronym. Print or type the authorized certifying official's name, title, and date. Finally, ensure that the certifying official signs or otherwise authenticates the form.
After completing the form, it must be transmitted to CNS Pantex, LLC via fax or email. Ensure that a copy accompanies the shipment for records and compliance purposes.
What You Should Know About This Form
What is a Certificate of Conformance (C of C)?
A Certificate of Conformance is a formal document that certifies that certain materials, items, or equipment conform to specific requirements stated in a Purchase Order or Contract. It indicates that the supplier has reviewed and complied with these requirements and that the information provided is accurate and complete. The certificate should accompany each shipment unless otherwise specified.
Why is the Certificate of Conformance important?
The importance of a Certificate of Conformance lies in its role in ensuring quality and compliance in the supply chain. It assures the buyer that the items delivered meet the necessary standards and specifications. This document helps to mitigate risks associated with noncompliance and nonconformance, ultimately fostering trust between suppliers and customers.
What information must be included in the Certificate of Conformance?
The Certificate of Conformance must contain several key pieces of information. This includes but is not limited to the Purchase Order or Contract number, the supplier's name and address, applicable codes and standards, item identification details, and any approved changes or exceptions. All sections of the form should be filled out accurately to avoid delays or issues during the shipment process.
Who is responsible for signing the Certificate of Conformance?
Signing the Certificate of Conformance is the responsibility of an Authorized Certifying Official from the supplier's organization. This official must be someone who has the authority to verify the information and attest to the compliance of the materials or items being delivered. Their signature verifies that all information is true and complete, ensuring accountability in the certification process.
What should a supplier do if there are nonconformances?
If there are nonconformances or deviations from the requirements, the supplier must not deliver or ship the materials until they have communicated these issues in writing to the Procurement Specialist. It is crucial that any changes or nonconforming conditions are identified and approved prior to shipment. This ensures clarity and compliance, preventing potential disputes or complications in the future.
Common mistakes
Filling out the Certificate of Conformance form requires careful attention to detail. One common mistake is omitting essential information in Section A. This section includes crucial data such as the Purchase Order (PO) number, revision number, and line item information. Each entry must be complete. Missing even a single piece of information could lead to delays or rejection of the shipment.
Another frequent error is failing to enter the correct Supplier Name and Address in Section B. This information must exactly match what is stated in the PO or contract. Discrepancies can cause confusion and impact the relationship between suppliers and purchasers.
In Section C, applicable requirements are vital. A mistake here would be not specifying the correct code or standard number. Each category must be complete with details, including revisions and editions. Leaving out this information undermines the certificate’s integrity and could lead to compliance issues.
People also often overlook the need for accuracy in the Certification Statement found in Section E. The authorized certifying official's name, title, and date must be precisely filled out. Misidentifying the official can invalidate the certification, making it essential to double-check this information.
It’s also crucial to accurately report any changes or deviations in Section D. Failing to document these appropriately can result in confusion or implications for compliance. Any approved changes must also reference the necessary documentation control numbers, ensuring clarity and traceability.
Lastly, neglecting to include an accompanying copy of the Certificate with the shipment is a significant oversight. While this may seem minor, without an accompanying form, the shipment may be delayed or returned, creating efficiency issues.
By being mindful of these common mistakes, suppliers can help ensure that their submissions are complete and compliant, ultimately leading to smoother transactions and improved relationships with procurement officials.
Documents used along the form
The Certificate of Conformance (C of C) is a critical document ensuring that products meet specified requirements. It is frequently accompanied by several other forms and documents that further facilitate compliance and quality assurance. Below is a list of commonly used forms alongside the Certificate of Conformance, each serving a distinct purpose in the procurement and delivery process.
- Purchase Order (PO): This document specifies the details of the transaction between a buyer and a seller, including items, quantities, prices, and delivery dates.
- Shipping Manifest: A comprehensive list of all items being shipped, along with their descriptions and quantities, used for tracking and verification.
- Inspection Report: A document that records the results of an inspection, detailing whether items meet specified standards before they are accepted.
- Quality Assurance Plan: A plan outlining the procedures and activities to ensure product quality, addressing testing methods and standards to be followed.
- Nonconformance Report: This form documents any deviations from expected quality or standards during production or inspection, providing a corrective action plan.
- Test Data Report: This report contains results from various tests performed on products to verify that they meet specific standards and specifications.
- Supplier Evaluation Form: A tool used to assess a supplier's capabilities and performance, including quality, delivery reliability, and customer service.
- Compliance Statement: A declaration affirming that goods meet the relevant laws and industry regulations, ensuring that products are safe for use.
- Certificate of Compliance: This document certifies that products conform to applicable standards and specifications, often required by regulatory authorities.
Each of these forms plays an essential role in the overall process of ensuring that products comply with required standards. Together, they help streamline quality assurance efforts and maintain accountability throughout the supply chain.
Similar forms
- Certificate of Compliance (CoC): Similar to the Certificate of Conformance, a Certificate of Compliance attests that products or services meet specified standards. It serves a similar purpose in confirming adherence to regulations, albeit usually focused on compliance with legal or safety requirements rather than specific procurement details.
- Inspection Certificate: This document verifies that goods have been inspected and meet predefined standards before delivery to the buyer. Like the Certificate of Conformance, it assures buyers that the items are acceptable for use but focuses more on the results of an inspection rather than a supplier’s attestation.
- Test Report: A Test Report provides evidence that specific tests have been conducted on a product, detailing the results. While the Certificate of Conformance certifies compliance with overall requirements, the Test Report dives deeper, showcasing individual test outcomes.
- Quality Assurance (QA) Certificate: This certificate verifies that a supplier adheres to established quality assurance processes. Similar to the Certificate of Conformance, it emphasizes the supplier's commitment to quality, yet it often encompasses broader aspects of production and quality control beyond just specific orders.
Dos and Don'ts
Things You Should Do
- Review the form instructions thoroughly before starting.
- Ensure all required sections are completed accurately.
- Use the exact legal name of your company as stated in the Purchase Order.
- Provide a clear and detailed certification statement.
- Attach any necessary documentation to support your entries.
Things You Shouldn't Do
- Do not submit the form without checking for errors or omissions.
- Avoid using abbreviations or informal names for your company.
- Do not ignore the instructions related to nonconformance.
- Do not deliver any items if there are changes that have not been approved.
- Never sign the certificate without confirming all information is accurate and complete.
Misconceptions
Misconceptions about the Certificate of Conformance (C of C) can lead to confusion and mismanagement in the supply chain. Here are seven common misconceptions clarified:
- The C of C is optional. Some believe that the C of C is not mandatory for all shipments. In reality, the completion of a C of C is a fundamental requirement for compliance with the Purchase Order or Contract. It ensures all items meet specified standards.
- One C of C covers multiple shipments. Many assume that one Certificate of Conformance can apply to multiple shipments. However, each shipment requires its own C of C, addressing each line item for the corresponding purchase order.
- The supplier can ignore nonconformance issues. There's a notion that suppliers can just overlook any nonconformances. This is not true; suppliers must report any deviations, substitutions, or nonconforming conditions in the specified section of the C of C before shipment.
- Filling out the C of C is simple. Some might think that completing a C of C is straightforward and doesn’t require careful attention. On the contrary, every entry must be precise and reflective of the actual items and conditions, requiring diligent review and compliance with all instructions.
- Only the authorized official needs to sign the C of C. There is a misconception that only the signature of the authorized certifying official matters. However, all provided information must also be accurate and complete, reflecting the responsibilities of the entire supplier organization.
- The C of C does not need to include supporting documents. Some people believe that the C of C stands alone. However, it is often necessary to attach additional documentation that validates the conformance of the items, particularly when changes or deviations are present.
- The C of C is meaningless if there are no issues. Others may think that if all items are conforming, the C of C does not need much attention. However, even when there are no issues, a C of C is critical for maintaining industry standards and ensuring traceability.
Understanding these misconceptions can help streamline processes, ensuring that both suppliers and customers can maintain compliance and quality in their transactions.
Key takeaways
Key Takeaways for Completing the Certificate of Conformance Form:
- Review Instructions: Thoroughly read the form instructions before filling out any sections. It's crucial to understand the requirements first.
- Complete Every Entry: Ensure all applicable fields, particularly in Sections A, B, C, D, and E, are accurately filled out. Incomplete forms can cause delivery delays.
- Certification Accuracy: The certifying official must attest that all information is truthful and complete. Misrepresentations can lead to serious consequences.
- Attach Supporting Documents: If using a system-generated C of C, make sure it meets the criteria outlined in the Pantex guidelines. Attach all necessary supporting documents with your submission.
Browse Other Templates
Metro Mobility Bus - All data provided should reflect your current health status and transportation needs accurately.
Social Security Earnings Request Form,Earnings Information Request F4,Form for Earning Details,SSA Earnings Statement Application,Request for Earnings Record,Social Security Earnings Inquiry Form,SSA Earnings Certification Application,Earnings Inform - Careful attention to detail helps prevent delays in processing the request.
Vics Bol - List the customer order number for better tracking on your end.