Fill Out Your Cigna And Remicade Form
The Cigna and Remicade form is a crucial document in the process of obtaining prior authorization for the infusion of Remicade (infliximab), a medication commonly used to treat various autoimmune conditions such as rheumatoid arthritis, Crohn's disease, and chronic plaque psoriasis. This form is designed to collect essential patient and provider information, ensuring that all necessary details are documented accurately to avoid any delays in processing. It requires input from healthcare providers regarding patient history, current treatment plans, and previous responses to therapy. The form includes inquiries about the patient's current weight, previous Remicade usage, and responses to other medications. Specific sections assess the treatment history related to various conditions and evaluate the patient's progress based on standardized measures. Providers must complete this form entirely, as incomplete submissions may lead to adverse determinations or delays in approval. Furthermore, it lays out the requirements for where the medication will be obtained, whether through a specialty pharmacy or retail outlet, and emphasizes the importance of secure communication of the authorization decision. Accessing necessary resources and further guidance can be found through the provided pharmacy services contact details, ensuring a streamlined process for both patients and providers.
Cigna And Remicade Example
Pharmacy Services
Phone:
Fax:
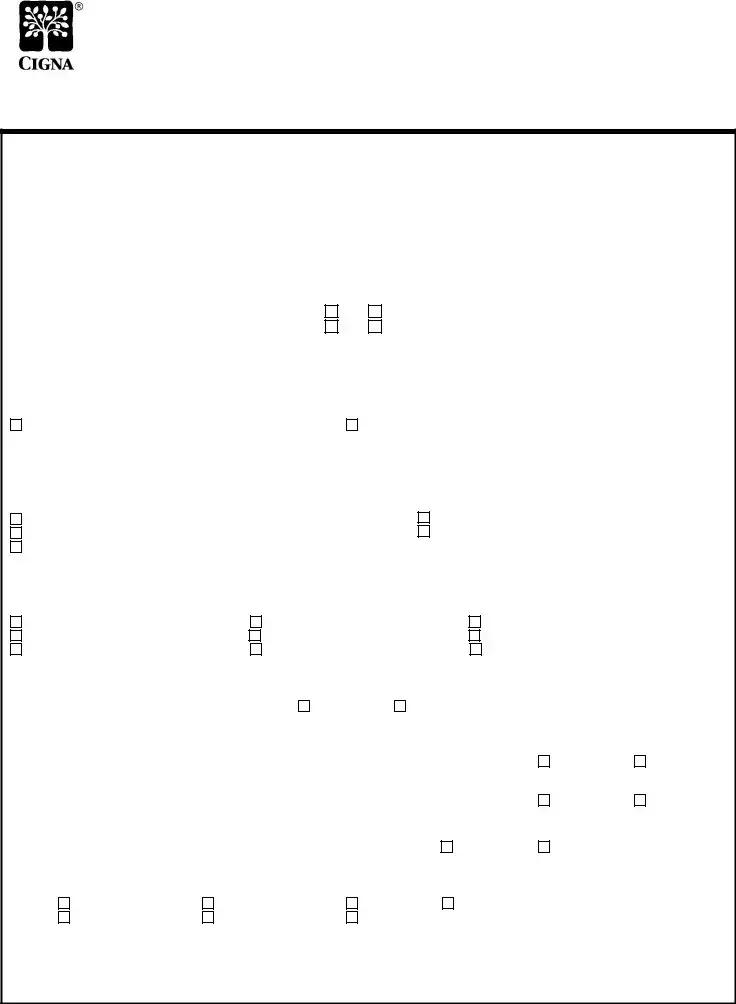
CIGNA HealthCare Prior Authorization Form
- Remicade (infliximab) -
Notice: Failure to complete this form in its entirety may result in delayed
processing or an adverse determination for insufficient information.
|
|
PROVIDER INFORMATION |
|
|
PATIENT INFORMATION |
|
|
|||||
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Provider Name: |
|
|
|
|
**Due to privacy regulations we will not be able to |
|
||||
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
respond via fax with the outcome of our review unless all |
|
||||
|
|
Specialty: |
|
* DEA or TIN: |
|
|
|
|||||
|
|
|
|
|
asterisked (*) items on this form are completed** |
|
||||||
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Office Contact Person: |
|
|
|
|
* Patient Name: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Office Phone: |
|
|
|
|
* CIGNA ID: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Office Fax: |
|
|
|
|
* Date Of Birth: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Is your fax machine kept in a secure location? |
Yes |
No |
* Patient Street Address: |
|
|
|
||||
|
|
|
|
|
|
|
|
|||||
|
|
* May we fax our response to your office? |
Yes |
No |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Office Street Address: |
|
|
|
|
City |
State |
Zip |
|
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
City |
State |
Zip |
|
Patient Phone: |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Medication requested:
Remicade (infliximab) 100mg vial |
|
Other (please specify): |
|
|
|
||
Dose and Quantity: |
Duration of therapy: |
|
|
|
|
||
Frequency of administration: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Where will this medication be obtained? |
|
|
|
|
|
|
|
CIGNA |
Retail pharmacy |
|
|
|
|||
Prescriber’s office stock (billing on a medical claim form) |
Home Health / Home Infusion vendor |
|
|
||||
Other (please specify): |
|
|
|
|
|
|
|
*If you wish to order this medication from CIGNA |
|||||||
|
|
|
|
|
|
||
Diagnosis related to use (please specify): |
|
|
|
|
|
||
Rheumatoid Arthritis |
|
Psoriatic Arthritis |
|
Active Ankylosing Spondylitis |
|
|
|
Chronic Plaque Psoriasis |
|
Ulcerative Colitis |
|
Crohn’s disease |
|
|
|
Fistulizing Crohn’s disease |
|
Inflammatory Bowel Disease Arthritis |
Other (please specify): |
|
|
||
What is the patient’s current weight? |
|
|
|
|
|
|
|
Has this patient been on Remicade in the past? |
Yes |
No |
|
|
|
|
|
If YES, what was the previous dosage? |
|
|
|
|
|
||
Does the patient have history of beneficial clinical response to Remicade (infliximab) therapy? |
Yes |
No |
|||||
|
|
|
|
|
|
|
|
Psoriatic or Reactive Arthritis: |
|
|
|
|
|
|
|
Does patient have evidence of failure, intolerance or contraindication to Methotrexate therapy? |
Yes |
No |
|||||
|
|
|
|
|
|
|
|
Rheumatoid Arthritis: |
|
|
|
|
|
|
|
Will this medication be used in combination with Methotrexate therapy? |
Yes |
|
No |
|
|
||
Please indicate if the patient has had evidence of failure, inadequate response, intolerance or contraindication to any of the following
|
|||
Methotrexate |
Azathioprine |
Gold |
Hydroxychloroquine |
Penacillamine |
Sulfasalazine |
Other (please specify): |
|
(Continued on page 2)
CIGNA HealthCare Prior Authorization Form – Remicade – Page 1 of 2
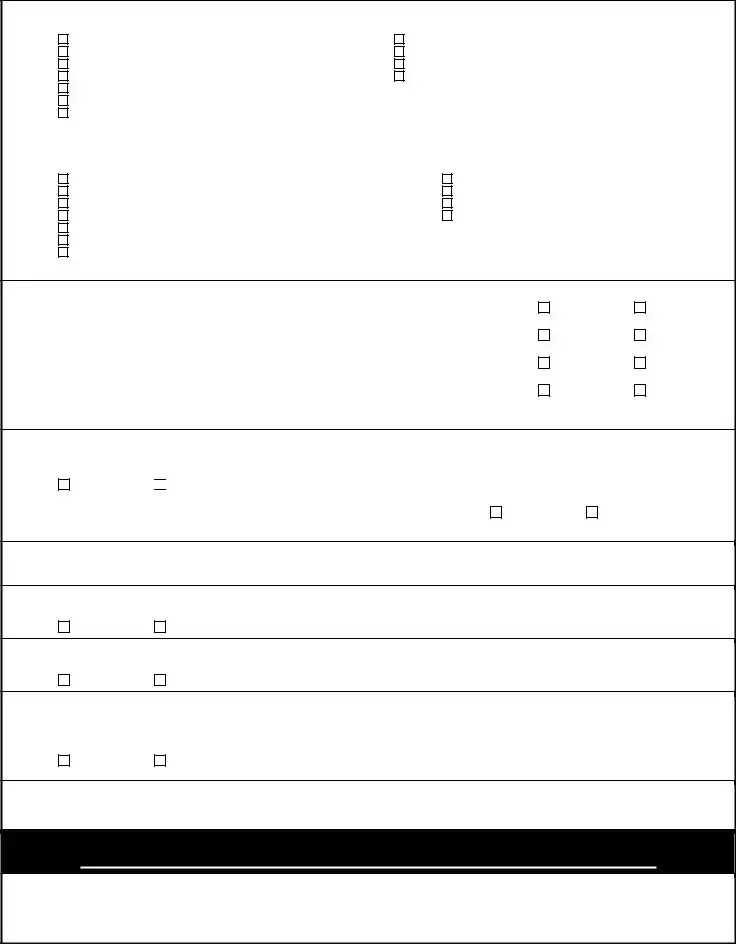
Which of the following methods was used to measure the patient’s disease progression PRIOR to therapy on Remicade? (Check all that apply):
Health Assessment Questionnaire Disease Index |
Visual Analogue scale (VAS) |
Likert scales of global response to pain by the patient/doctor |
Global Arthritis Score (GAS) |
Clinical Disease Activity Index (CDAI) |
Simplified Disease Activity Index (SDAI) |
Progression of radiographic damage of involved joints |
Disease Activity Scale (DAS) score |
Disease Activity Score based on
Elevation of ESR (> 28 mm/hr), or
Other (please specify) :
If this is a request for CONTINUED THERAPY (after at least 16 weeks of treatment), has the patient shown beneficial response to treatment with Remicade based on any of the following measurements? (Check all that showed a beneficial response to Remicade therapy):
Health Assessment Questionnaire Disease Index |
Visual Analogue scale (VAS) |
Likert scales of global response to pain by the patient/doctor |
Global Arthritis Score (GAS) |
Clinical Disease Activity Index (CDAI) |
Simplified Disease Activity Index (SDAI) |
Disease Activity Scale (DAS) score |
ESR or |
Disease Activity Score based on
At least a 20% improvement according to ACR 20% response criteria
Other (please specify) :
Chronic Plaque Psoriasis:
Does the patient have history of beneficial clinical response to Remicade (infliximab) therapy?
Is the patient a candidate for systemic therapy?
Is the severity great enough that the patient is a candidate for Photo Therapy?
Is this a request for a renewal of a previously granted authorization?
If YES, please document improvement since beginning therapy:
Yes
Yes
Yes
Yes
No
No
No
No
Crohn’s Disease:
Has the patient had failure, contraindication, or intolerance to conventional therapies such as aminosalicylate, corticosteroids, or immunomodulators?
Yes 
 No
No
Did the patient have a failure or intolerance to adalimumab (Humira) therapy?
Yes
No
Fistulizing Crohn’s Disease:
How long have fistulas persisted?
Inflammatory Bowel Disease Arthritis:
Has the patient had failure, contraindication, or intolerance to sulfasalazine, azathioprine, steroids, or, methotrexate?
Yes |
No |
Ankylosing Spondylitis:
Has the patient had failure, contraindication, or intolerance to
Yes |
No |
Ulcerative colitis:
Has the patient had failure, contraindication, or intolerance to conventional therapies such as corticosteroids (e.g, prednisone, methylprednisolone),
Yes |
No |
If YES, please specify which medications: |
Additional pertinent information:
CIGNA HealthCare’s coverage position on this and other medications may be viewed online at:
http://www.cigna.com/customer_care/healthcare_professional/coverage_positions
Please fax completed form to
Our standard response time for prescription drug coverage requests is
“CIGNA Pharmacy Management” or “CIGNA HealthCare” refer to various operating subsidiaries of CIGNA Corporation. Products and services |
V 041610 |
|
|
are provided by these subsidiaries and not by CIGNA Corporation. These subsidiaries include Connecticut General Life Insurance Company, Tel- |
|
Drug, Inc., |
|
CIGNA HealthCare Prior Authorization Form - Remicade - Page 2 of 2
Form Characteristics
| Fact Name | Details |
|---|---|
| Provider Information | This section requires the provider's name, specialty, DEA or TIN, office contact person, and contact numbers. |
| Patient Information | Patient details include name, CIGNA ID, date of birth, address, and phone number. |
| Medication Requested | Remicade (infliximab) is the primary medication requested along with options for dosage, quantity, and duration. |
| Prior Authorization Requirement | The form must be completed in its entirety to avoid delays or adverse determinations. |
| Therapy Metrics | Evaluations like health assessments and disease activity scores are necessary to measure the patient’s condition prior to therapy. |
| Previous Treatment History | Questions determine if the patient has had prior use of Remicade and their response to it. |
| Diagnosis Information | Possible diagnoses include rheumatoid arthritis, psoriatic arthritis, Crohn’s disease, and others. |
| State-Specific Forms | Verification regarding adherence to local regulations is advised when filling out forms for specific states. |
| Submission Guidelines | Completed forms must be faxed to CIGNA using the designated fax number: (800)390-9745. |
| Response Time | CIGNA standard response time for coverage requests ranges from 2 to 4 business days. |
Guidelines on Utilizing Cigna And Remicade
The following steps will guide you through the process of completing the Cigna and Remicade prior authorization form. Ensure that all required fields are filled out accurately to avoid delays in processing. Once the form is completed, fax it to the provided number.
- Begin by filling out the Provider Information section. Include the provider's name, specialty, DEA or TIN, office contact person, phone number, and fax number.
- Complete the Patient Information section. Fill in the patient’s name, CIGNA ID, date of birth, street address, city, state, zip code, and phone number.
- Indicate whether the provider's fax machine is kept in a secure location by selecting "Yes" or "No".
- Specify if the response can be faxed to the provider's office by selecting "Yes" or "No".
- In the Medication Requested section, select Remicade (infliximab) or specify another medication. Fill in the dosage, quantity, duration of therapy, J-Code, and frequency of administration.
- Choose the location where the medication will be obtained from the provided options.
- State the diagnosis related to the use of the medication and indicate the patient's current weight.
- Answer the questions regarding the patient’s history with Remicade and any previous dosages.
- Check "Yes" or "No" for relevant questions regarding the patient’s response to Methotrexate therapy and other non-responding medications.
- List the methods used to measure the patient's disease progression prior to therapy by checking all applicable options.
- If applicable, check the measurements indicating a beneficial response to Remicade therapy.
- Complete any additional questions regarding specific conditions, including Crohn’s disease, chronic plaque psoriasis, and ulcerative colitis.
- Provide any additional pertinent information relevant to the authorization.
- Once filled out completely, fax the form to the designated number (800)390-9745.
What You Should Know About This Form
What is the purpose of the Cigna and Remicade form?
The Cigna and Remicade form is designed for healthcare providers to obtain prior authorization for the use of Remicade (infliximab) for their patients. This process ensures that the prescribed medication is appropriate and covered under the patient's health plan. Completing the form thoroughly helps to avoid delays in processing and ensures the patient receives timely access to their medication.
What information is required on the form?
The form requires detailed information about both the healthcare provider and the patient. Essential items include the provider's name, specialty, contact information, as well as the patient's name, date of birth, CIGNA ID, and medication details. Incomplete forms may lead to delays or denials due to lack of sufficient information.
What should I do if I have not received a response after submitting the form?
If you have not received a response within the standard timeframe of 2 to 4 business days, it is advisable to contact Cigna's Pharmacy Services directly. By calling their helpline at (800) 244-6224, you can inquire about the status of your request and ensure that it is being processed promptly.
Can I fax the completed form, and what are the requirements for doing so?
Yes, the completed form should be faxed to (800) 390-9745. It's important to ensure that all required fields marked with an asterisk (*) are filled out completely before faxing. In addition, confirm that your fax machine is secure, as Cigna adheres to strict privacy regulations and cannot disclose outcomes via fax if the form is incomplete.
What if the patient has been on Remicade before?
If the patient has previously received Remicade, the form requires information about their past dosage and whether they experienced a beneficial clinical response. This information helps in determining if the past treatment was successful and supports the case for ongoing therapy.
Are there any specific health conditions that are covered under this authorization?
Yes, the form specifies various health conditions for which Remicade may be prescribed, including rheumatoid arthritis, ulcerative colitis, Crohn's disease, and ankylosing spondylitis, among others. It is essential to specify the patient's diagnosis related to the use of Remicade in the form to support the request for authorization.
Common mistakes
Completing the Cigna and Remicade prior authorization form is a critical step in securing medication coverage. However, common mistakes can hinder the process. One frequent error is incomplete information. Every section marked with an asterisk (*) is mandatory. Missing any of these fields can lead to processing delays or denials of coverage. It's essential to ensure that the provider and patient details, including the patient's date of birth and CIGNA ID, are accurately filled in.
Another common mistake involves failing to provide the correct diagnosis information. The form requires a specific diagnosis related to the use of Remicade. If the chosen diagnosis does not match the patient's medical history or if it’s listed as 'Other' without a clarification, it could raise questions during the review process. Always make sure to reference the patient's official diagnosis from their medical records.
Additionally, not detailing the patient’s treatment history can lead to complications. Questions regarding past therapies, dosage information, and previous responses to Remicade must be answered thoroughly. If a patient has previously received Remicade, it's crucial to state their past dosage and confirm if they had any beneficial responses. This information supports the necessity for the continued use of the medication.
Another area of confusion is the disease progression assessments. The form requests various methods used to measure the patient’s condition prior to Remicade treatment. Failing to check relevant assessments may cause reviewers to question the patient's need for the medication. Clear documentation of the effectiveness of previous treatments provides a strong rationale for therapy continuation and helps prevent unnecessary denial.
Finally, neglecting to provide additional information can be detrimental. The last section allows you to include any pertinent information that may support the request. Even if it seems insignificant, providing context about the patient’s condition, previous therapies, and physician recommendations can make a difference in the authorization decision. Consider all angles of the patient's treatment journey when completing this form to ensure a successful outcome.
Documents used along the form
The Cigna and Remicade form is a vital document for patients seeking prior authorization for Remicade (infliximab). However, several other forms and documents are commonly utilized in conjunction with this form to facilitate the prior authorization process, manage patient care, and ensure compliance with insurance protocols. Below is a list of these documents, along with concise descriptions of their purposes.
- Patient Information Form: This form collects essential information about the patient, including demographic details and insurance information, often needed for insurance claims and prior authorizations.
- Medication History Report: This document provides an overview of the patient’s medication history, highlighting previous treatments, dosages, and responses, which aids in determining the appropriateness of Remicade therapy.
- Prior Authorization Request Form: Used generally for medications, this form requests approval from the insurance provider before the prescribed medication can be dispensed, ensuring coverage under a patient's plan.
- Clinical Evaluation Form: This form gathers clinical data and assessments from healthcare providers, demonstrating the medical necessity for the requested treatment or medication.
- Consents and Acknowledgments: These forms obtain patient consent for sharing medical information with their insurer, ensuring compliance with privacy regulations and facilitating communication.
- Drug Utilization Review (DUR) Form: This document reviews the patient's drug therapy and assesses the appropriateness of prescribed medications based on clinical guidelines and individual patient needs.
- Side Effect Reporting Form: This form documents any side effects experienced by the patient during treatment, which may impact the decision-making process regarding continued use of Remicade.
- Insurance Coverage Policy Document: This document outlines the specific criteria for coverage relating to Remicade and any related procedures, helping providers understand insurance limitations.
- Discharge Summary or Referral Letters: Often provided by the healthcare provider, these documents summarize the patient’s treatment journey and may include specialist referrals relevant to the medication prescribed.
- Appeals Form for Denied Authorization: Should prior authorization be denied, this form is used to formally appeal the decision, providing additional information or clarifications necessary to support the request.
Utilizing these complementary documents alongside the Cigna and Remicade form can significantly enhance the likelihood of securing approval and ensuring effective treatment planning for patients requiring Remicade therapy.
Similar forms
The Cigna and Remicade form aligns closely with several other healthcare-related documents that share similar purposes, such as obtaining prior authorizations for prescriptions. Here are four comparable documents:
- Prior Authorization Form for Specialty Medications: This document requests approval from insurance for costly specialty drugs requiring comprehensive review. Like the Remicade form, it needs detailed patient information and justification for the prescribed medication, providing evidence of the condition and treatment plans.
- Medication Prior Authorization Request Form: This form is commonly utilized to get prior approval for various medications prior to commencement of therapy. Similar to the Cigna Remicade form, it also includes sections for patient demographics, prescriber details, medication specifics, and previous treatment history.
- Clinical Trial Enrollment Form: Individuals considering participation in a clinical trial often complete this form. It includes patient information, medical history, and the specific intervention being considered. Like the Remicade form, it gathers vital data to ensure appropriateness for the requested care.
- Healthcare Coverage Determination Request: Patients or providers may submit this request for clarity on what is covered under a healthcare plan. Mirroring the Cigna and Remicade form, it requires thorough patient details and specifics about the treatment to determine coverage eligibility.
Dos and Don'ts
When filling out the Cigna and Remicade form, there are several actions that can improve the process and others that may hinder it. Following these recommendations can help ensure a smooth and efficient experience.
- Do: Complete all required fields marked with an asterisk (*) to prevent delays.
- Do: Provide accurate and up-to-date patient and provider information.
- Do: Include the patient's diagnosis and relevant medical history in detail.
- Do: Indicate if the patient has received Remicade previously, along with prior dosage information.
- Don't: Leave any mandatory fields blank, as this may result in an incomplete submission.
- Don't: Neglect to check the secure fax location box if applicable; this is important for privacy.
- Don't: Use abbreviations or jargon that may be unclear, ensuring clarity in communication.
- Don't: Delay submission. If urgent, call Pharmacy Services to expedite processing.
Misconceptions
Misconceptions about the Cigna and Remicade authorization form can lead to confusion and delays in treatment. Here are ten common misconceptions, along with explanations to clarify the facts:
- Completing the form isn’t important. Some people believe that minor omissions won’t affect the process. However, failing to complete all required fields can result in delays or even denial of the request.
- It’s okay to skip details about the patient's history. Skipping a patient's medical history, including past treatments with Remicade, can lead to an incomplete picture for the approval committee. Providing comprehensive details is crucial.
- Any pharmacy can dispense Remicade without issue. Not all pharmacies can supply Remicade. It’s important to specify whether the medication will be obtained from a specialty pharmacy, retail pharmacy, or the prescriber’s office.
- Weight doesn’t matter when requesting medication. The patient's weight is often a consideration for dosing and can impact approval. It must be included in the form to ensure the correct dosage is evaluated.
- Previous treatment responses are irrelevant. The patient's past experiences with Remicade or similar medications provide essential context for the current request. Indicating responses to previous treatments can help justify the need for therapy.
- All physicians can fax approval outcomes. Due to privacy regulations, Cigna often cannot send fax approvals unless all required fields are filled out, hindering communication if the form is incomplete.
- Documentation of disease progression is optional. Evidence of disease progression should be documented using approved assessment methods. This information is crucial for supporting the request.
- Renewals of only certain types of therapies require different forms. Regardless of whether it's a new request or a renewal, the form must be filled out completely to evaluate the authorization accurately.
- An urgent request is treated the same as a regular one. Urgent requests require an expedited process. It's essential to communicate urgency directly to Pharmacy Services for quicker handling.
- Cigna’s coverage position can be disregarded. Cigna's coverage positions are crucial and should be reviewed beforehand. Understanding what is covered can streamline the process and minimize frustrations.
By addressing these misconceptions, individuals can better navigate the authorization process for Remicade with Cigna, ensuring timely and effective treatment.
Key takeaways
Filling out the Cigna and Remicade form is essential for smooth processing. Here are some key takeaways to keep in mind:
- Complete all required fields. Leaving any asterisked (*) items blank may cause delays.
- Ensure the provider and patient information is accurate and current. Incorrect details can lead to complications.
- Consider the patient’s medical history with Remicade. Previous dosages and responses are important for approval.
- Include any other medications the patient has tried. Listing failures or intolerances helps establish need.
- Measure disease progression carefully. Use accepted methods to document the patient's condition before therapy begins.
- For continued therapy requests, indicate the patient's response back to Remicade. Evidence of improvement is crucial for renewal.
- Send the completed form via fax to the designated number along with any additional information required.
Timely submission can significantly impact treatment outcomes for patients.
Browse Other Templates
How Much Do You Have to Owe in Child Support to Go to Jail - Providing health insurance information can support the child support claim.
Ohio Medicaid Forms - The Ohio JFS 02390 form is used to authorize skilled tasks completed by Home Care Attendants.
Transcript Release Form,Academic Record Request,Student Academic Transcript Application,Official Transcript Request,Student Transcript Authorization Form,Transcript Requisition Document,College Transcript Application,Request for Transcript Release,So - Provide a valid phone number for any necessary clarifications regarding your request.
