Fill Out Your Clia Application Cms 116 Form
The CLIA Application CMS 116 form is essential for laboratories seeking certification under the Clinical Laboratory Improvement Amendments (CLIA). This form covers various key aspects, including the laboratory’s general information, the type of certification being requested, and the laboratory’s performance capabilities. For instance, applicants must provide basic details such as facility name, federal tax identification number, and effective date. Additionally, the form requires clarity on the type of certificate sought—whether for waived testing, Provider Performed Microscopy Procedures (PPM), compliance, or accreditation. Each of these categories specifies different assembly of sections that must be completed. Moreover, the CMS 116 form also asks for information about the laboratory type, operational hours, and whether the facility operates multiple sites under specific regulatory exceptions. Testing capabilities are broken down into waived testing, PPM testing, and non-waived testing, requiring laboratories to enumerate all the tests they conduct and projected annual volumes. Ownership types are examined along with potential affiliations of laboratory directors with other certified facilities. This structured approach ensures that laboratories meet stringent standards necessary to ensure reliable testing and patient safety.
Clia Application Cms 116 Example
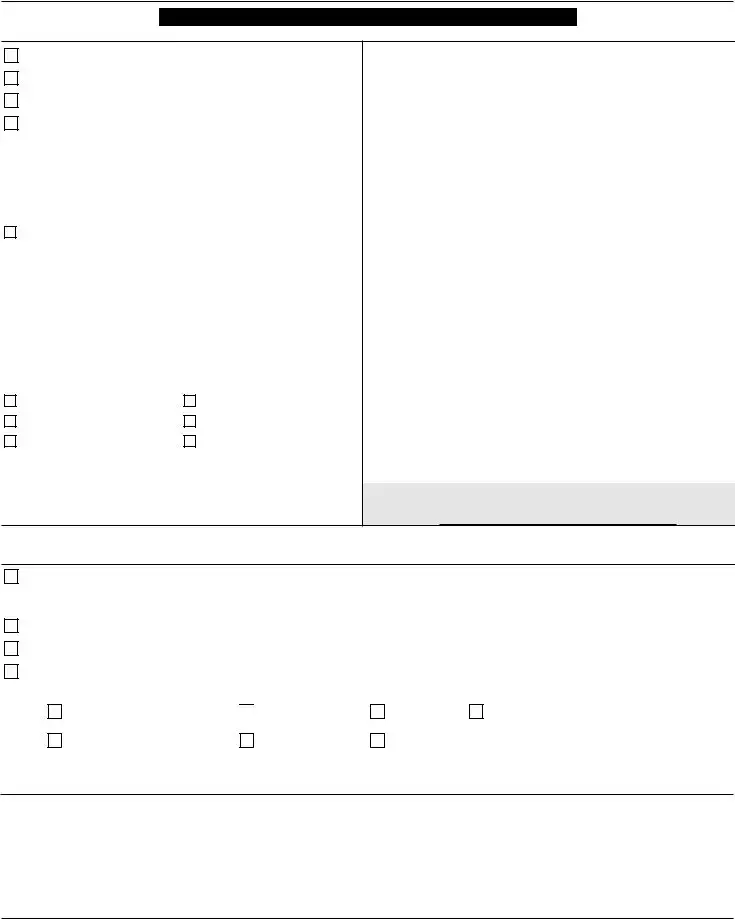
DEPARTMENT OF HEALTH AND HUMAN SERVICES |
Form Approved |
CENTERS FOR MEDICARE & MEDICAID SERVICES |
OMB No. |
|
|
CLINICAL LABORATORY IMPROVEMENT AMENDMENTS (CLIA)
APPLICATION FOR CERTIFICATION
ALL APPLICABLE SECTIONS OF THIS FORM MUST BE COMPLETED.
I. GENERAL INFORMATION
Initial Application |
Anticipated Start Date |
|
CLIA IDENTIFICATION NUMBER |
|
|
|
|||||||||
|
|
|
|
|
|
|
|||||||||
Survey |
|
|
|
|
|
|
|
|
|
D |
|
|
|
|
|
Change in Certificate Type |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
(If an initial application leave blank, a number will be assigned) |
|||||||||
|
|
|
|
|
|
|
|
|
|
||||||
Other Changes (Specify) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Effective Date |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FACILITY NAME |
|
|
|
|
|
|
|
|
FEDERAL TAX IDENTIFICATION NUMBER |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EMAIL ADDRESS |
|
|
|
|
|
|
|
|
TELEPHONE NO. (Include area code) |
FAX NO. (Include area code) |
|||||
RECEIVE FUTURE NOTIFICATIONS VIA EMAIL |
|
|
|
|
|
|
|
|
|
|
|
||||
FACILITY ADDRESS — Physical Location of Laboratory (Building, Floor, Suite if |
|
MAILING/BILLING ADDRESS (If different from facility address) send Fee Coupon |
|||||||||||||
applicable.) Fee Coupon/Certificate will be mailed to this Address unless mailing |
|
or certificate |
|
|
|
||||||||||
or corporate address is specified |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NUMBER, STREET (No P.O. Boxes) |
|
|
|
|
|
|
NUMBER, STREET |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CITY |
|
|
STATE |
ZIP CODE |
|
CITY |
STATE |
ZIP CODE |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SEND FEE COUPON TO THIS ADDRESS |
SEND CERTIFICATE TO THIS ADDRESS |
|
CORPORATE ADDRESS (If different |
NUMBER, STREET |
|
||||||||||
PICK ONE: |
|
|
PICK ONE: |
|
|
|
|
|
from facility) send Fee Coupon or |
|
|
|
|||
|
|
|
|
|
|
|
certificate |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Physical |
|
|
Physical |
|
|
|
|
|
|
|
|
|
|
|
|
Mailing |
|
|
Mailing |
|
|
|
|
|
CITY |
STATE |
ZIP CODE |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Corporate |
|
|
Corporate |
|
|
|
|
|
|
|
|||||
NAME OF DIRECTOR (Last, First, Middle Initial) |
|
|
|
|
|
Laboratory Director’s Phone Number |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CREDENTIALS |
|
|
|
|
|
|
|
|
FOR OFFICE USE ONLY |
|
|
|
|||
Date Received
II.TYPE OF CERTIFICATE REQUESTED (Check only one) Please refer to the accompanying instructions for inspection and certificate testing requirements)
Certificate of Waiver (Complete Sections I – VI and IX – X)
NOTE: Laboratory directors performing
Certificate for Provider Performed Microscopy Procedures (PPM) (Complete Sections
Certificate of Compliance (Complete Sections I – X)
Certificate of Accreditation (Complete Sections I – X) and indicate which of the following organization(s) your laboratory is accredited by for CLIA purposes, or for which you have applied for accreditation for CLIA purposes.
The Joint Commission
CAP

 AAHHS/HFAP
AAHHS/HFAP
COLA
AABB
ASHI
A2LA
If you are applying for a Certificate of Accreditation, you must provide evidence of accreditation for your laboratory by an approved accreditation organization as listed above for CLIA purposes or evidence of application for such accreditation within 11 months after receipt of your Certificate of Registration.
PRA Disclosure Statement
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is
Form |
1 |
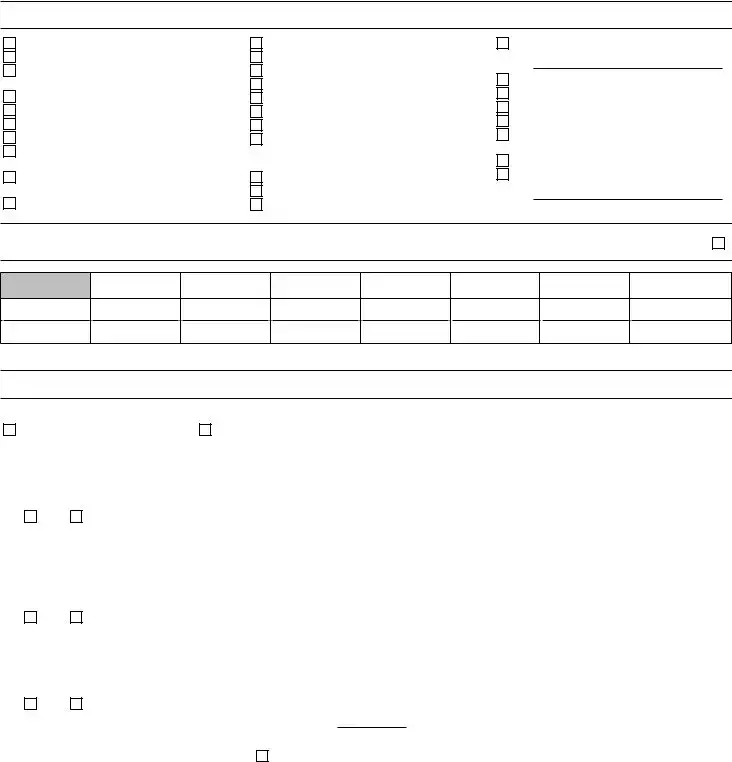
III. TYPE OF LABORATORY (Check the one most descriptive of facility type)
01 Ambulance
02Ambulatory Surgery Center
03Ancillary Testing Site in Health Care Facility
04Assisted Living Facility
05Blood Bank
06Community Clinic
07Comp. Outpatient Rehab Facility
08End Stage Renal Disease Dialysis Facility
09Federally Qualified Health Center
10Health Fair
11Health Main. Organization
12Home Health Agency
13Hospice
14Hospital
15Independent
16Industrial
17Insurance
18Intermediate Care Facilities for Individuals with Intellectual Disabilities
19Mobile Laboratory
20Pharmacy
21Physician Office
22Practitioner Other (Specify)
23Prison
24Public Health Laboratories
25Rural Health Clinic
26School/Student Health Service
27Skilled Nursing Facility/ Nursing Facility
28Tissue Bank/Repositories
29Other (Specify)
IV. HOURS OF LABORATORY TESTING (List times during which laboratory testing is performed in HH:MM format) If testing 24/7 Check Here
SUNDAY |
MONDAY |
TUESDAY WEDNESDAY THURSDAY |
FRIDAY |
SATURDAY |
FROM:
TO:
(For multiple sites, attach the additional information using the same format.)
V. MULTIPLE SITES (must meet one of the regulatory exceptions to apply for this provision in
Are you applying for a single site CLIA certificate to cover multiple testing locations?
No. If no, go to section VI.
Yes. If yes, complete remainder of this section.
Indicate which of the following regulatory exceptions applies to your facility’s operation.
1.Is this a laboratory that is not at a fixed location, that is, a laboratory that moves from testing site to testing site, such as mobile unit providing laboratory testing, health screening fairs, or other temporary testing locations, and may be covered under the certificate of the designated primary site or home base, using its address?
Yes
No
If yes and a mobile unit is providing the laboratory testing, record the vehicle identification number(s) (VINs) and attach to the application.
2.Is this a
multiple sites?
Yes
No
If yes, provide the number of sites under the certificate |
|
and list name, address and test performed for each |
site below. |
|
|
3.Is this a hospital with several laboratories located at contiguous buildings on the same campus within the same physical location or street address and under common direction that is filing for a single certificate for these locations?
Yes
No
If yes, provide the number of sites under this certificateand list name or department, location within hospital and specialty/subspecialty areas performed at each site below.
If additional space is needed, check here
and attach the additional information using the same format.
|
NAME AND ADDRESS/LOCATION |
TESTS PERFORMED/SPECIALTY/SUBSPECIALTY |
|
|
|
|
|
NAME OF LABORATORY OR HOSPITAL DEPARTMENT |
|
||
|
|
|
|
ADDRESS/LOCATION (Number, Street, Location if applicable) |
|
||
|
|
|
|
CITY, STATE, ZIP CODE |
|
TELEPHONE NO. (Include area code) |
|
|
|
|
|
NAME OF LABORATORY OR HOSPITAL DEPARTMENT |
|
||
|
|
||
ADDRESS/LOCATION (Number, Street, Location if applicable) |
|
||
|
|
|
|
CITY, STATE, ZIP CODE |
|
TELEPHONE NO. (Include area code) |
|
|
|
|
|
|
|
|
|
Form |
2 |
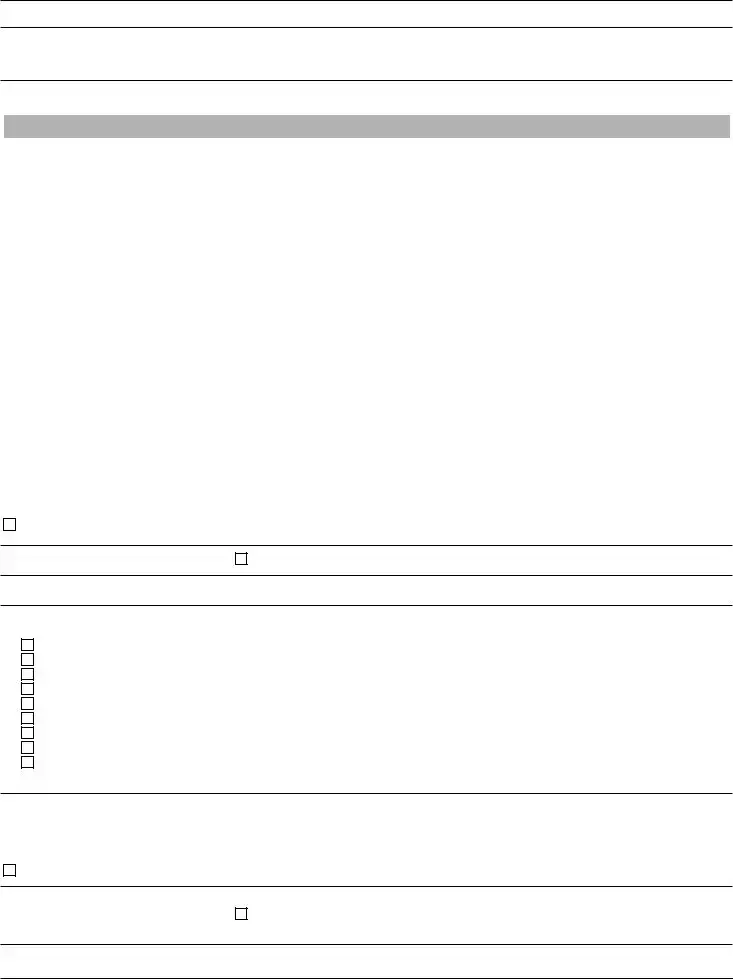
In the next three sections, indicate testing performed and estimated annual test volume.
VI. WAIVED TESTING If only applying for a Certificate of Waiver, complete this section and skip sections VII (PPM Testing) and VIII
Identify the waived testing (to be) performed by completing the table below. Include each analyte, test system, or device used in the laboratory.
|
ANALYTE / TEST |
TEST NAME |
MANUFACTURER |
|
|
Example: Streptococcus group A |
Ace Rapid Strep Test |
Acme Corporation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indicate the ESTIMATED TOTAL ANNUAL TEST volume for all waived tests performed ________________
Check if no waived tests are performed
If additional space is needed, check here
and attach additional information using the same format.
VII. PPM TESTING If only applying for a Certificate for PPM, complete this section and skip section VIII
Listed below are the only PPM tests that can be performed by a facility having a Certificate for PPM. Mark the checkbox by each PPM procedure(s) to be performed.
Direct wet mount preparations for the presence or absence of bacteria, fungi, parasites, and human cellular elements
Potassium hydroxide (KOH) preparations
Pinworm examinations
Fern tests
Urine sediment examinations
Nasal smears for granulocytes
Fecal leukocyte examinations
Qualitative semen analysis (limited to the presence or absence of sperm and detection of motility)
Indicate the ESTIMATED TOTAL ANNUAL TEST volume for all PPM tests performed ________________
If also performing waived complexity tests, complete Section VI. For laboratories applying for certificate of compliance or certificate of accreditation, also include PPM test volume in the specialty/subspecialty category and the “total estimated annual test volume” in section VIII.
Check if no PPM tests are performed
If additional space is needed, check here
and attach additional information using the same format.
Form |
3 |
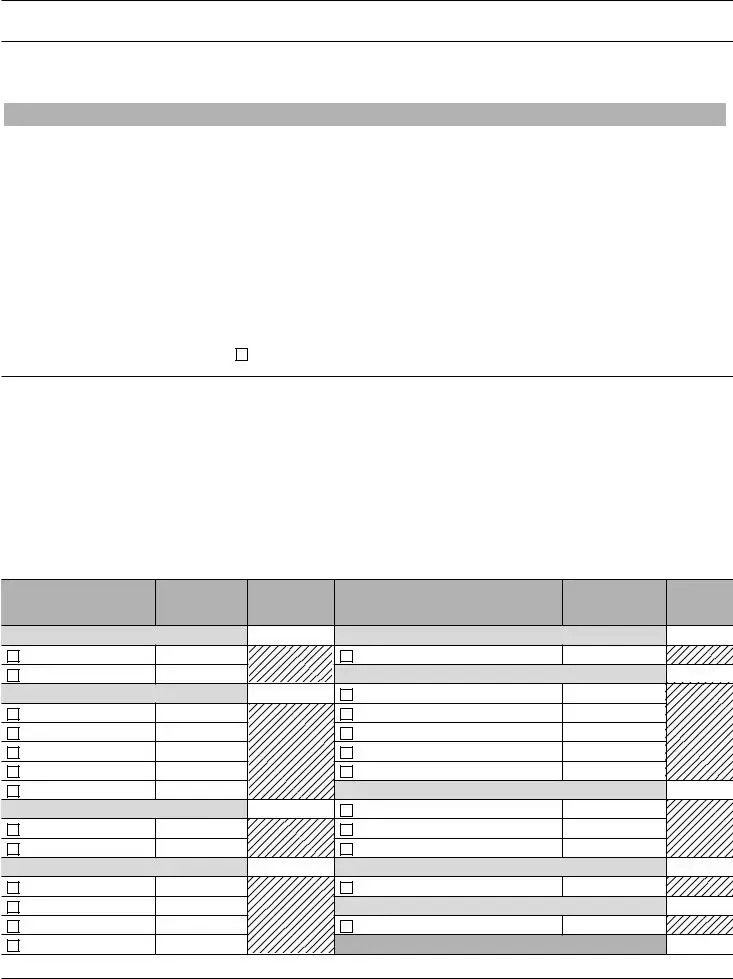
VIII.
Identify the
|
ANALYTE / TEST |
TEST NAME |
MANUFACTURER |
M or H |
|
|
Example: Potassium |
Quick Potassium Test |
Acme Lab Corporation |
M |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If additional space is needed, check here
and attach additional information using the same format.
If you perform testing other than or in addition to waived tests, complete the information below. If applying for one certificate for multiple sites, the total volume should include testing for ALL sites.
If additional space is needed, check here and attach additional information using the same format.” Include text box similar to Section VII.
Place a check (3) in the box preceding each specialty/subspecialty in which the laboratory performs testing. Enter the
estimated annual test volume for each specialty. Do not include testing not subject to CLIA, waived tests, or tests run for quality control, calculations, quality assurance or proficiency testing when calculating test volume. (For additional guidance on counting test volume, see the instructions included with the application package.)
If applying for a Certificate of Accreditation, indicate the name of the Accreditation Organization beside the applicable specialty/ subspecialty for which you are accredited for CLIA compliance. (The Joint Commission, AAHHS/HFAP, AABB, A2LA ,CAP, COLA or ASHI)
SPECIALTY / |
ACCREDITING |
ANNUAL |
SPECIALTY / |
ACCREDITING |
ANNUAL |
|
TEST |
||||||
SUBSPECIALTY |
ORGANIZATION TEST VOLUME |
SUBSPECIALTY |
ORGANIZATION |
|||
VOLUME |
||||||
|
|
|
|
|
||
HISTOCOMPATIBILITY 010 |
|
|
HEMATOLOGY 400 |
|
|
|
Transplant |
|
|
Hematology |
|
|
|
Nontransplant |
|
|
IMMUNOHEMATOLOGY |
|
|
|
MICROBIOLOGY |
|
|
ABO Group & Rh Group 510 |
|
|
|
Bacteriology 110 |
|
|
Antibody Detection (transfusion) 520 |
|
|
|
Mycobacteriology 115 |
|
|
Antibody Detection (nontransfusion) 530 |
|
|
|
Mycology 120 |
|
|
Antibody Identification 540 |
|
|
|
Parasitology 130 |
|
|
Compatibility Testing 550 |
|
|
|
Virology 140 |
|
|
PATHOLOGY |
|
|
|
DIAGNOSTIC IMMUNOLOGY |
|
|
Histopathology 610 |
|
|
|
Syphilis Serology 210 |
|
|
Oral Pathology 620 |
|
|
|
General Immunology 220 |
|
|
Cytology 630 |
|
|
|
CHEMISTRY |
|
|
RADIOBIOASSAY 800 |
|
|
|
Routine 310 |
|
|
Radiobioassay |
|
|
|
Urinalysis 320 |
|
|
CLINICAL CYTOGENETICS 900 |
|
|
|
Endocrinology 330 |
|
|
Clinical Cytogenetics |
|
|
|
Toxicology 340 |
|
|
TOTAL ESTIMATED ANNUAL TEST VOLUME: |
|
||
Form |
|
|
|
|
4 |
|

IX. TYPE OF CONTROL (CHECK THE ONE MOST DESCRIPTIVE OF OWNERSHIP TYPE)
VOLUNTARY NONPROFIT

 01 Religious Affiliation
01 Religious Affiliation

 02 Private Nonprofit
02 Private Nonprofit

 03 Other Nonprofit
03 Other Nonprofit
(Specify)
FOR PROFIT

 04 Proprietary
04 Proprietary
GOVERNMENT

 05 City
05 City

 06 County
06 County

 07 State
07 State

 08 Federal
08 Federal

 09 Other Government
09 Other Government
(If 09 is selected, please specify the country
or the province.)
Does this facility have partial or full ownership by a foreign entity or foreign government?  Yes
Yes  No
No
If Yes, what is the country of origin for the foreign entity?
X. DIRECTOR AFFILIATION WITH OTHER LABORATORIES
If the director of this laboratory serves as director for additional laboratories that are separately certified, please complete the following:
CLIA NUMBER
NAME OF LABORATORY
ATTENTION: READ THE FOLLOWING CAREFULLY BEFORE SIGNING APPLICATION
Any person who intentionally violates any requirement of section 353 of the Public Health Service Act as amended or any regulation promulgated thereunder shall be imprisoned for not more than 1 year or fined under title
18, United States Code or both, except that if the conviction is for a second or subsequent violation of such a requirement such person shall be imprisoned for not more than 3 years or fined in accordance with title 18, United States Code or both.
Consent: The applicant hereby agrees that such laboratory identified herein will be operated in accordance with applicable standards found necessary by the Secretary of Health and Human Services to carry out the purposes of section 353 of the Public Health Service Act as amended. The applicant further agrees to permit the Secretary, or any Federal officer or employee duly designated by the Secretary, to inspect the laboratory and its operations and its pertinent records at any reasonable time and to furnish any requested information or materials necessary to determine the laboratory’s eligibility or continued eligibility for its certificate or continued compliance with CLIA requirements.
PRINT NAME OF DIRECTOR OF LABORATORY
PRINT NAME OF OWNER OF LABORATORY
SIGNATURE OF OWNER/DIRECTOR OF LABORATORY (SIGN IN INK OR USE A SECURE ELECTRONIC SIGNATURE)
DATE
NOTE: Completed 116 applications must be sent to your local State Agency. Do not send any payment with your completed 116 application.
STATE AGENCY CONTACT INFORMATION CAN BE FOUND AT:
Form |
5 |

THE CLINICAL LABORATORY IMPROVEMENT AMENDMENTS (CLIA) APPLICATION
(FORM
INSTRUCTIONS FOR COMPLETION
CLIA requires every facility that tests human specimens for the purpose of providing information for the diagnosis, prevention or treatment of any disease or impairment of, or the assessment of the health of, a human being to meet certain Federal requirements.
If your facility performs tests for these purposes, it is considered, under the law, to be a laboratory. Facilities only collecting or preparing specimens (or both) or only serving as a mailing service are not considered laboratories. CLIA does not apply to a facility that only performs forensic testing. CLIA applies even if only one or a few basic tests are performed, and even if you are not charging for testing. In addition, the CLIA legislation requires financing of all regulatory costs through fees assessed to affected facilities.
The CLIA application (Form
NOTE: WAIVED TESTS ARE NOT EXEMPT FROM CLIA. FACILITIES PERFORMING ONLY THOSE TESTS CATEGORIZED AS WAIVED MUST APPLY FOR A CLIA CERTIFICATE OF WAIVER.
NOTE: Laboratory directors performing
•Verification of State Licensure, as applicable
•Documentation of qualifications:
•Education (copy of Diploma, transcript from accredited institution, CMEs),
•Credentials, and
•Laboratory experience.
Individuals who attended foreign schools must have an evaluation of their credentials determining equivalency of their education to education obtained in the United States. Failure to submit this information will delay the processing of your application.
ALL APPLICABLE SECTIONS MUST BE COMPLETED. INCOMPLETE APPLICATIONS CANNOT BE PROCESSED AND WILL BE RETURNED TO THE FACILITY. PRINT LEGIBLY OR TYPE INFORMATION.
I. GENERAL INFORMATION
For an initial applicant, check “initial application”. For an initial survey or for a recertification, check “survey”. For a request to change the type of certificate, check “change in certificate type” and provide the effective
date of the change. For all other changes, including change in location, director, lab closure, etc., check “other changes” and provide the effective date of the change.
CLIA Identification Number: For an initial applicant, the CLIA number should be left blank. The number will be assigned when the application is processed. For all other applicants, enter the 10 digit CLIA identification number already assigned and listed on your CLIA certificate.
Facility Name: Be specific when indicating the name of your facility, particularly when it is a component of a larger entity, e.g., respiratory therapy department in XYZ Hospital. For a physician’s office, this may be the name of the physician. NOTE: the information provided is what will appear on your certificate.
Email Address: A valid Email Address is optional and will be used for communications between the CLIA program and the laboratory. Selecting the RECEIVE NOTIFICATIONS VIA EMAIL checkbox, requires the laboratory to enter a valid Email Address.
Physical Facility Address: This address is mandatory and must reflect the physical location where the laboratory testing is performed. The address may include a floor, suite and/or room location, but cannot be a Post Office box or Mail Stop.
If the laboratory has a separate mailing and/or corporate address (from the Facility Address), please complete the appropriate sections on the form.
Mailing Address: This address is optional and may be used if the laboratory wants to direct the mailing of the CLIA fee coupon and/or CLIA certificate to an alternate location, such as an accounts payable office. A Post Office box number or Mail Stop number may be used as part of the Mailing Address for this section.
Corporate Address: This address is optional and may be used if the laboratory wants to direct the mailing of the CLIA fee coupon and/or CLIA certificate to another location, such as, the main headquarters or home office for the laboratory. A Post Office box number or Mail Stop number may be used as part of the Corporate Address for this section.
Form Mailing: Select the address (Physical, Mailing, Corporate) where the CLIA fee coupon and CLIA certificate are to be mailed.
For Office Use Only: The date received is the date the form is received by the state agency or CMS regional office for processing.
II. TYPE OF CERTIFICATE REQUESTED
Select your certificate type based on the highest level of test complexity performed by your laboratory. A laboratory performing
When completing this section, please remember that a facility holding a:
Form |
Instructions |
•Certificate of Waiver can only perform tests categorized as waived;*
•Certificate for Provider Performed Microscopy Procedures (PPM) can only perform tests categorized as PPM, or tests categorized as PPM and waived tests;*
•Certificate of Compliance can perform tests categorized as waived, PPM and moderate and/or high complexity tests provided the applicable CLIA quality standards are met following a CLIA survey; and
•Certificate of Accreditation can perform tests categorized as waived, PPM and moderate and/ or high complexity
*A current list of waived and PPM tests may be obtained from your State agency. Specific test system categorizations can also be found on the Internet at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/ cfCLIA/clia.cfm.
III. TYPE OF LABORATORY
Select the type that is most descriptive of the location where the laboratory testing is performed.
If selecting ‘mobile laboratory’ (code 19), a mobile laboratory is defined as a movable,
If selecting ‘Practitioner Other’ (code 22), this type includes practitioners such as, dentists, chiropractors, etc.
IV. HOURS OF ROUTINE OPERATION
Provide only the times when actual laboratory testing is performed in your facility. Please use the HH:MM
format and check box marked ‘24/7’ if laboratory testing is performed continuously, e.g., 24 hours a day, 7 days a week. Do not use military time.
V. MULTIPLE SITES
You can only qualify for the multiple site provision (more than one site under one certificate) if you meet one of the CLIA requirements described in 42 CFR 493.
VI. WAIVED TESTING
Indicate the estimated total annual test volume for all waived tests performed. List can be found at: https:www.cms.gov/CLIA/downloads/waivetbl.pdf
VII. PPM TESTING
Indicate the estimated total annual test volume for all PPM tests performed. List can be found at: https://www.cms.gov/CLIA/downloads/ppmplist.pdf
VIII.
The total Estimated Annual Test volume in this section includes all
IX. TYPE OF CONTROL
Select the type of ownership or control which most appropriately describes your facility.
X. DIRECTOR OF ADDITIONAL LABORATORIES List all other facilities for which the director is responsible and that are under different certificates. Note that for a Certificate of PPM, Certificate of Compliance or Certificate of Accreditation, an individual can only serve as the director for no more than five certificates.
Reminders - Before submitting the Form
1.Include the current or estimated annual test volume.
2.For Certificate for PPM, Certificate of Compliance, or Certificate of Accreditation, include the laboratory director qualifications.
3.Do not send any money with your application.
4.Send the completed Form
Once the completed Form
If you need additional information concerning CLIA, or if you have questions about completion of this form, please contact your State agency. State agency contact information can be found at:
Form |
Instructions |

VIII.
TESTS COMMONLY PERFORMED AND THEIR CORRESPONDING
LABORATORY SPECIALTIES/SUBSPECIALITIES
HISTOCOMPATIBILITY (010)
HLA Typing (disease associated antigens)
MICROBIOLOGY
Bacteriology (110)
Gram Stain Culture Susceptibility Strep screen
Antigen assays (H.pylori, Chlamydia, etc.)
Mycobacteriology (115)
Acid Fast Smear
Mycobacterial culture
Mycobacterial susceptibility
Mycology (120)
Fungal Culture
DTM
KOH Preps
Parasitology (130)
Direct Preps
Ova and Parasite Preps
Wet Preps
Virology (140)
RSV (Not including waived kits)
HPV assay
Cell culture
DIAGNOSTIC IMMUNOLOGY
Syphilis Serology (210)
RPR
FTA, MHATP
General Immunology (220)
Allergen testing
ANA Antistreptolysin O
Antigen/Antibody (hepatitis, herpes, rubella, etc.) Complement (C3, C4)
Immunoglobulin
HIV
Mononucleosis assay Rheumatoid factor
Tumor marker (AFP, CA
*Tumor markers can alternatively be listed under Routine Chemistry instead of General Immunology.
HEMATOLOGY (400)
Complete Blood Count (CBC) WBC count
RBC count Hemoglobin
Hematocrit (Not including spun micro) Platelet count
Differential
Activated Clotting Time
Prothrombin time (Not including waived instruments) Partial thromboplastin time
Fibrinogen Reticulocyte count
Manual WBC by hemocytometer Manual platelet by hemocytometer Manual RBC by hemocytometer Sperm count
IMMUNOHEMATOLOGY
ABO group (510)
Rh(D) type (510)
Antibody screening
Antibody identification (540)
Compatibility testing (550)
PATHOLOGY
Dermatopathology
Oral Pathology (620)
PAP smear interpretations (630)
Other Cytology tests (630)
Histopathology (610)
RADIOBIOASSAY (800)
Red cell volume
Schilling test
CLINICAL CYTOGENETICS (900)
Fragile X Buccal smear
FISH studies for: neoplastic disorders, congenital disorders or solid tumors.
Form |
Instructions |

CHEMISTRY
Routine Chemistry (310)
Albumin
Ammonia
Alk Phos
ALT/SGPT
AST/SGOT
Amylase
Bilirubin
Blood gas (pH, pO2, pCO2)
BUN Calcium Chloride Cholesterol Cholesterol, HDL CK/CK isoenzymes CO2 Creatinine Ferritin
Folate
GGT
Glucose (Not fingerstick) Iron
LDH/LDH isoenzymes Magnesium Potassium
Protein, electrophoresis Protein, total
PSA Sodium Triglycerides Troponin Uric acid Vitamin B12
Endocrinology (330)
Cortisol
HCG (serum pregnancy test) T3
T3 Uptake
T4
T4, free
TSH
Toxicology (340)
Acetaminophen
Blood alcohol
Blood lead (Not waived)
Carbamazepine
Digoxin
Ethosuximide
Gentamicin
Lithium
Phenobarbital
Phenytoin
Primidone
Procainamide
NAPA
Quinidine
Salicylates
Theophylline
Tobramycin
Therapeutic Drug Monitoring
Urinalysis** (320)
Automated Urinalysis (Not including waived instruments) Microscopic Urinalysis
Urine specific gravity by refractometer Urine specific gravity by urinometer Urine protein by sulfosalicylic acid
** Dipstick urinalysis is counted in Section VI. WAIVED TESTING
NOTE: This is not a complete list of tests covered by CLIA. Other
Form |
Instructions |

GUIDELINES FOR COUNTING TESTS FOR CLIA
•For chemistry, each
•For clinical cytogenetics, the number of tests is determined by the number of specimen types processed on each patient; e.g., a bone marrow and a venous blood specimen received on one patient is counted as
two tests. NOTE: For all other genetic tests, the number of tests is determined by the number of results reported in the final report.
•For manual gynecologic and nongynecologic cytology, each slide (not case) is counted as one test.
•For flow cytometry, each measured individual analyte (e.g. T cells, B cells, CD4, etc.) that is ordered and reported should be counted separately.
•For general immunology, testing for allergens should be counted as one test per individual allergen.
•Genetics tests should be placed in the specialty or subspecialty where they fit best, according to the methodology of the test.
•For hematology, each measured individual analyte of a complete blood count or flow cytometry test that is ordered and reported is counted separately. The WBC differential is counted as one test.
•For histocompatibility, each HLA typing (including disease associated antigens) is counted as one test, each HLA antibody screen is counted as one test and each HLA cross match is counted as one test. For example, a
•For histopathology, each block (not slide) is counted as one test. Autopsy services are not included. For those laboratories that perform special stains on histology slides, the test volume is determined by adding the number of special stains performed on slides to the total number of specimen blocks prepared by the laboratory.
•For immunohematology, each ABO, Rh, antibody screen, crossmatch or antibody identification is counted as one test.
•For microbiology, susceptibility testing is counted as one test per group of antibiotics used to determine sensitivity for one organism. Cultures are counted as one per test request from each specimen regardless of the extent of identification, number of organisms isolated, and number of tests/procedures required for identification. Each gram stain or
•For urinalysis, microscopic and macroscopic examinations, each count as one test. Macroscopics (dipsticks) are counted as one test regardless of the number of reagent pads on the strip.
•For all specialties/subspecialities, do not count calculations (e.g., A/G ratio, MCH, T7, etc.), quality control, quality assurance, or proficiency testing assays.
If you need additional information concerning counting tests for CLIA, please contact your State agency.
Form |
Instructions |
Form Characteristics
| Fact Name | Description |
|---|---|
| Purpose | The CMS-116 form is used for the Clinical Laboratory Improvement Amendments certification application. |
| Application Types | It can be used for initial applications, changes in certificate type, or other changes. |
| Required Sections | All applicable sections must be completed to ensure proper processing of the application. |
| CLIA Identification Number | A unique CLIA identification number is assigned upon initial application. |
| Testing Certificate Types | Four types of certificates can be requested: Waiver, PPM, Compliance, and Accreditation. |
| Director Qualifications | Laboratory directors must meet specific qualifications for non-waived testing under CLIA regulations. |
| Contact Information | Applicants must provide thorough contact details, including email and telephone information. |
| Testing Hours | The form requires listing the hours during which laboratory testing is performed. |
| Accreditation Requirements | If applying for a Certificate of Accreditation, proof of accreditation is necessary within 11 months after registration. |
| State-Specific Laws | The application may be subject to state-specific regulations, which can be found by consulting local State Agencies. |
Guidelines on Utilizing Clia Application Cms 116
Filling out the CLIA Application CMS 116 form is a crucial step for laboratories seeking certification. Once the application is completed, it will need to be submitted to the appropriate state agency for review. Be sure to provide accurate information and double-check the details to avoid delays in processing.
- Start by selecting the type of application you are submitting: Initial Application, Change in Certificate Type, or Other Changes.
- Enter the facility's information, including the name, federal tax identification number, email address, phone number, and fax number.
- Provide the physical address of the laboratory and any different mailing or corporate address where notifications will be sent.
- State the name of the laboratory director and their contact number.
- Choose the type of certificate you are requesting by checking the appropriate box, following the specified sections for completion.
- Indicate the type of laboratory by checking the one that best describes the facility.
- Record the hours of laboratory testing performed during the week using the specified format.
- If applying for a certificate covering multiple sites, indicate whether you meet the regulatory exceptions and provide the necessary details for each location.
- Complete the section for waived testing by listing each type of test performed and the estimated total annual test volume for these tests.
- If applicable, list the PPM tests performed and their estimated annual test volume.
- For non-waived testing, provide a detailed list of tests performed, including analysis and estimated annual test volume.
- Check the box that describes the facility's ownership type.
- If the laboratory director is affiliated with other laboratories, fill out the details for each one.
- Finally, read the consent statement carefully before signing and dating the application.
What You Should Know About This Form
What is the CLIA Application CMS 116 form used for?
The CLIA Application CMS 116 form is used to apply for certification under the Clinical Laboratory Improvement Amendments (CLIA). This form is essential for laboratories that wish to perform tests on human specimens. It has various sections that collect important information about the laboratory, such as its location, type of services offered, and the specific types of certificates being requested. This form ensures that the laboratory meets the federal standards necessary for operation.
Who needs to fill out this form?
Any laboratory or facility that plans to conduct diagnostic testing on human specimens must complete this form to obtain a CLIA certificate. This includes a wide range of facilities, such as hospitals, clinics, independent laboratories, and certain offices of healthcare providers. If the laboratory is applying for a certificate for the first time or if it is changing its certificate type, this form is required.
What are the different types of certificates that can be requested on this form?
There are several types of certificates that can be requested on the CLIA Application CMS 116 form. These include the Certificate of Waiver, which allows for certain simple tests; the Certificate for Provider-Performed Microscopy Procedures (PPM); the Certificate of Compliance, which confirms adherence to CLIA regulations; and the Certificate of Accreditation, available for laboratories accredited by specific organizations. Each type has its specific requirements and coverage areas.
What information is required to complete this application?
The application requires detailed information about the laboratory, including its name, location, and federal tax identification number. You will also need to provide specifics about the type of testing performed, the laboratory director's credentials, and the ownership structure of the facility. Accurate completion of each section is crucial for the timely processing of the application and to avoid any delays.
Where should the completed form be submitted?
Once you have completed the CLIA Application CMS 116 form, it must be submitted to your local State Agency. It is important not to send any payment with the application. You can find the appropriate contact information for your State Agency at the Centers for Medicare & Medicaid Services (CMS) website. Ensuring that you direct your submission to the right place is essential for the processing of your application.
Common mistakes
Filling out the CLIA Application CMS 116 form can be a detailed process, and many applicants encounter common pitfalls that can delay their certification. One significant mistake occurs when individuals fail to provide all applicable sections of the form. The instructions clearly state that every section must be completed, even if some areas seem irrelevant to their lab. Leaving sections blank can lead to immediate rejection of the application.
Another frequent error is not accurately reporting the facility address and mailing address. Applicants should ensure that the exact physical location of their laboratory is provided. Omitting crucial details such as suite numbers or failing to include zip codes can cause issues later when the certificate or fee coupon is mailed. Additionally, ensuring that these addresses are identical on all documentation is vital to avoid confusion.
A third mistake is misidentifying the type of certificate required for their laboratory operations. There are various certificates available, including Certificates of Waiver, PPM, Compliance, and Accreditation. Applicants must take the time to review which types apply to their laboratory's operations. Choosing the wrong certificate can lead to unnecessary delays and complications in the application process.
Lastly, many people underestimate the importance of signature requirements. The form mandates signatures from both the laboratory director and the owner. Applications missing these signatures may be returned for correction, thus extending the time to obtain certification. Taking time to double-check for signatures before submitting can save applicants both time and frustration.
Documents used along the form
The Clinical Laboratory Improvement Amendments (CLIA) Application CMS 116 form is essential for laboratories seeking certification to operate in the United States. This application enables regulatory bodies to assess the compliance of laboratories with federal standards and ensures the safety and accuracy of laboratory testing. In addition to the CMS 116 form, various other documents and forms may be required to complete the certification process and maintain compliance.
- CMS 117 Form: This form is utilized to update information regarding any changes in laboratory ownership or director. Laboratories must complete this form to maintain accurate records with regulatory authorities.
- Certificate of Accreditation: For laboratories seeking a certificate based on accreditation, this document proves that they meet the standards set by an approved accrediting organization. This is necessary for labs pursuing higher complexity testing.
- Operations Manual: Each laboratory should have an operations manual detailing standard operating procedures, policies, and protocols. This document plays a key role during inspections and ensures laboratories adhere to quality standards.
- Inspection Reports: These reports outline findings from the most recent on-site inspections. They show compliance status and illustrate any areas requiring corrective action.
- Staff Competency Records: Documenting staff credentials and training is vital. These records should provide evidence that all personnel meet the necessary qualifications for their respective positions.
- Quality Control Logs: Laboratories must maintain logs showing quality control measures in place. These logs are essential for demonstrating adherence to testing standards and ongoing quality assurance.
- Proficiency Testing Results: Participation in proficiency testing is mandatory for compliance with CLIA. Laboratories must keep records of their results to demonstrate competency in their testing procedures.
- Annual Report: Some laboratories may need to submit an annual report summarizing testing volumes and compliance activities. This helps regulatory bodies monitor ongoing laboratory performance.
- State-specific Applications: If applicable, some states require additional state-level applications or forms. These documents ensure that laboratories comply with both federal and state regulations.
- Fee Coupons: Along with the CMS 116 form, fee coupons serve as proof of payment for applicable certification fees. Submission of this document is critical for successful application processing.
Having the above documents prepared and readily available can streamline the certification process and help laboratories maintain compliance with CLIA regulations. For each form and document, accuracy and completeness are essential in ensuring that laboratories provide safe and reliable testing services to the public.
Similar forms
- CLIA Certificate of Waiver Application: Similar to the CLIA Application CMS 116 form, this document also requests information related to the type of tests being performed. It requires details on the waived testing procedures and specifics about the lab’s capabilities.
- CLIA Certificate for Provider Performed Microscopy (PPM): Like the CMS 116, this application focuses specifically on PPM tests and mandates that laboratories provide their testing details and volume similar to the information required in the CMS 116 form.
- CLIA Certificate of Compliance Application: This application shares a similar structure with the CMS 116 form. Both require comprehensive information on testing categories, compliance with regulations, and the director’s qualifications and affiliations.
- CMS 855A Application for Enrollment in Medicare: This document is akin to the CMS 116 in that it collects detailed information about healthcare facilities. It includes sections on ownership, services provided, and compliance, paralleling the requirements found in the CLIA application.
Dos and Don'ts
When filling out the CLIA Application CMS 116 form, it's important to ensure accuracy and compliance. Here are seven key do's and don'ts to guide you through the process:
- Do ensure all applicable sections of the form are completed to avoid delays.
- Do double-check the information provided for accuracy, including the facility name and tax identification number.
- Do submit any required proof of qualifications for the laboratory director, especially for non-waived testing.
- Do provide clear contact information, including email and telephone numbers, for future correspondence.
- Don't leave any sections blank unless instructed to do so. Each part is essential for processing.
- Don't forget to specify the type of certificate you are applying for; this determines the sections you need to complete.
- Don't send your application to the PRA Reports Clearance Office; instead, submit it to your local state agency.
Misconceptions
Misunderstandings about the CLIA Application CMS 116 form are common. Below are ten misconceptions, each followed by an explanation to clarify the facts.
- It’s only for large laboratories. Many believe the form is exclusively for large facilities. In reality, it applies to all laboratory types, regardless of size, including smaller entities like physician offices and community clinics.
- The application process is the same for all types of certificates. Some assume the process is uniform. Different certificates—such as Certificate of Waiver versus Certificate of Compliance—require specific sections to be completed, emphasizing the need for thorough reading of the instructions.
- You cannot operate until approved. A common thought is that operations must cease during the submission of the application. However, laboratories can continue testing while their application is under review, provided they comply with all relevant regulations.
- All tests performed need to be listed on the application. Many people think every single test must be documented. Instead, applicants only need to detail tests subject to CLIA requirements, which simplifies reporting.
- It’s unnecessary to have proof of qualifications for laboratory directors. Some may ignore this requirement, but the law mandates that laboratory directors performing non-waived tests must prove their qualifications, including education and training.
- Paying a fee ensures quick approval. There's a belief that a payment guarantees rapid action on an application. Approval timelines actually depend on various factors, including completeness of the application and the responsiveness of local state agencies.
- The application can be sent to any CMS office. Some individuals mistakenly think any regional CMS office will suffice. It’s crucial to send the application to the appropriate local State Agency, as specified in the instructions.
- You can use a P.O. Box for the laboratory address. Some applicants assume a P.O. Box is acceptable for the facility address. However, a physical address is required for compliance with CLIA regulations.
- There’s no need to submit additional documentation. Another misconception is that the form alone suffices. Depending on the certificate type chosen, applicants may need to provide evidence of accreditation or qualifications alongside the form.
- Application mistakes are easily fixable later. Some think they can correct errors after submission without consequences. It's essential to ensure accuracy at the outset, as inaccuracies can lead to delays or even denial of the certificate.
Understanding these misconceptions can lead to a more straightforward and efficient application process. Clarity around these points can empower laboratories to ensure they meet compliance requirements while obtaining their CLIA certification.
Key takeaways
When completing the CLIA Application CMS 116 form, attention to detail is paramount. Here are six key takeaways to ensure accuracy and compliance:
- Complete All Sections: Every applicable section of the form must be filled out. Incomplete forms can lead to delays or rejections.
- Choose the Correct Certificate: Select the appropriate certificate type based on the laboratory's operations. This ranges from a Certificate of Waiver to a Certificate of Accreditation.
- Gather Necessary Documentation: If applying for non-waived testing, be prepared to submit proof of qualifications for the laboratory director along with the completed application.
- Identify Ownership Type: Clearly indicate the type of ownership for your laboratory, as this impacts compliance regulations.
- Provide Accurate Testing Information: List all tests performed accurately, including estimated annual test volumes, to avoid potential compliance issues.
- Submit to the Right Agency: Ensure the completed application is sent to the appropriate local State Agency. Do not include payment with the application.
Browse Other Templates
Humana Book - Check the product codes carefully while placing your order.
Dwc 69 Form - Doctor's certification on the DWC069 affirms compliance with Texas Labor Code regulations.
Lease Renewal Letter to Landlord - Revisions to lease agreements must be mutually agreed upon and documented.
