Fill Out Your Contingency Plan For Vaccine Storage Form
The Contingency Plan for Vaccine Storage form serves as a critical tool for healthcare facilities to ensure the safe transfer and storage of vaccines during emergencies. This form is designed to capture essential information, including the facility name, address, and points of contact responsible for managing vaccine transfers. It requires details such as the phone numbers of key clinic staff and backup personnel, which increases communication efficiency during urgent situations. The document outlines procedures for transferring vaccines, specifying whether these should go to another facility and if a generator is available. Essential aspects such as the inventory of vaccines and associated tracking numbers are also included. Additionally, checklists for transporting both refrigerated and frozen vaccines guide staff on the necessary temperature controls and proper handling, ensuring that all vaccines remain viable during transport. The form further emphasizes the importance of maintaining accurate records, including where to obtain necessary supplies like ice or dry ice. In the event of larger emergencies, such as city-wide evacuations, it directs facilities to contact their health service region for further guidance, highlighting a collaborative approach to public health challenges.
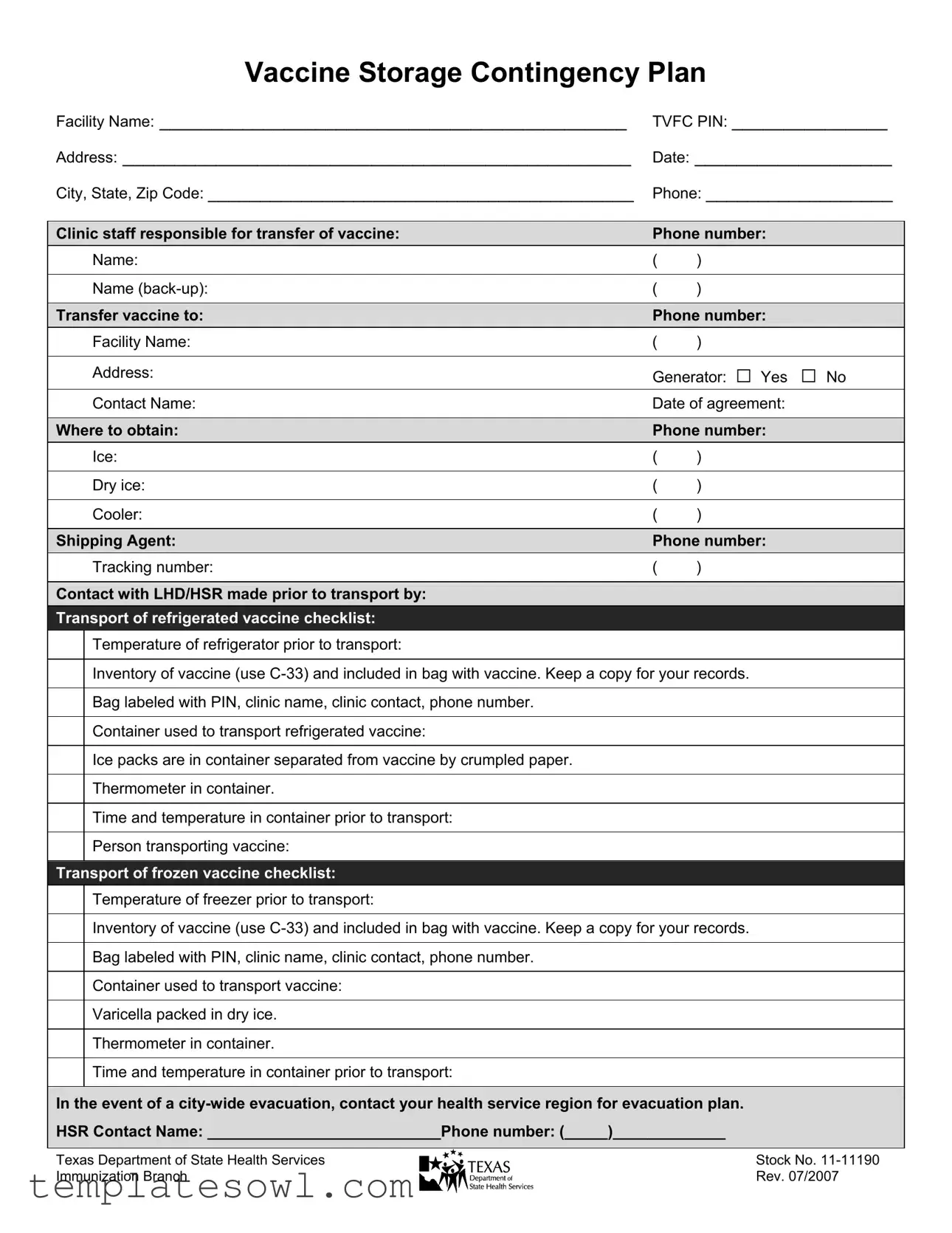
Contingency Plan For Vaccine Storage Example

Vaccine Storage Contingency Plan
Facility Name: _____________________________________________ |
|
TVFC PIN: _______________ |
||
Address: _________________________________________________ |
|
Date: ___________________ |
||
City, State, Zip Code: _________________________________________ |
|
Phone: __________________ |
||
|
|
|
|
|
Clinic staff responsible for transfer of vaccine: |
|
Phone number: |
||
|
Name: |
( |
) |
|
|
|
|
|
|
|
Name |
( |
) |
|
|
|
|
|
|
Transfer vaccine to: |
|
Phone number: |
||
|
Facility Name: |
( |
) |
|
|
|
|
|
|
|
Address: |
|
Generator: □ Yes □ No |
|
|
|
|
||
|
Contact Name: |
Date of agreement: |
||
|
|
|
|
|
Where to obtain: |
|
Phone number: |
||
|
Ice: |
( |
) |
|
|
|
|
|
|
|
Dry ice: |
( |
) |
|
|
|
|
|
|
|
Cooler: |
( |
) |
|
|
|
|
|
|
Shipping Agent: |
|
Phone number: |
||
|
Tracking number: |
( |
) |
|
|
|
|
|
|
Contact with LHD/HSR made prior to transport by: |
|
|
|
|
Transport of refrigerated vaccine checklist: |
|
|
|
|
|
Temperature of refrigerator prior to transport: |
|
|
|
|
|
|||
|
Inventory of vaccine (use |
|||
|
|
|
|
|
|
Bag labeled with PIN, clinic name, clinic contact, phone number. |
|
|
|
|
|
|
|
|
|
Container used to transport refrigerated vaccine: |
|
|
|
|
|
|
|
|
|
Ice packs are in container separated from vaccine by crumpled paper. |
|
|
|
|
|
|
|
|
|
Thermometer in container. |
|
|
|
|
|
|
|
|
|
Time and temperature in container prior to transport: |
|
|
|
|
|
|
|
|
|
Person transporting vaccine: |
|
|
|
|
|
|
|
|
Transport of frozen vaccine checklist: |
|
|
|
|
|
Temperature of freezer prior to transport: |
|
|
|
|
|
|||
|
Inventory of vaccine (use |
|||
|
|
|
|
|
|
Bag labeled with PIN, clinic name, clinic contact, phone number. |
|
|
|
|
|
|
|
|
|
Container used to transport vaccine: |
|
|
|
|
|
|
|
|
|
Varicella packed in dry ice. |
|
|
|
|
|
|
|
|
|
Thermometer in container. |
|
|
|
|
|
|
|
|
|
Time and temperature in container prior to transport: |
|
|
|
|
|
|
|
|
In the event of a
Texas Department of State Health Services |
Stock No. |
Immunization Branch |
Rev. 07/2007 |
Form Characteristics
| Fact Name | Description |
|---|---|
| Facility Name | The form requires the facility's name where the vaccines are stored. |
| TVFC PIN | A unique identification number assigned to the facility as part of the Texas Vaccines for Children program. |
| Contact Information | Includes the address, phone number, and additional contact details of the clinic. |
| Designated Staff | Clinics must list staff members responsible for vaccine transfer, along with their phone numbers. |
| Transfer Location | The form includes spaces for the name and contact information of the facility receiving the vaccines. |
| Ice and Cooler Inventory | Clinics must indicate the type of cooling agents available for transport, such as ice or dry ice. |
| Transport Checklist | A checklist ensures that critical conditions, such as temperature and inventory, are recorded prior to vaccine transport. |
| Emergency Protocol | The form provides instructions for contacting local health officials in case of a city-wide evacuation. |
| Container Specifications | Requirements for the type of container used for transporting vaccines, including insulation and thermometer placement. |
| Governing Laws | The form is regulated by the Texas Department of State Health Services, which outlines specific State laws related to vaccine handling and storage. |
Guidelines on Utilizing Contingency Plan For Vaccine Storage
Filling out the Contingency Plan For Vaccine Storage form requires gathering specific information related to your facility and vaccine transport plans. Ensure that all details are accurate, as this helps maintain the integrity of the vaccine during storage and transfer. Follow the provided steps below to complete the form correctly.
- Enter the Facility Name in the designated space.
- Input the TVFC PIN number.
- Provide the Address of the facility.
- Write the Date of filling out this form.
- Fill in the City, State, and Zip Code.
- Include the Phone number for the clinic.
- List the clinic staff responsible for vaccine transfer along with their phone number.
- Provide the name of a back-up staff member and their phone number.
- Identify the Facility Name where the vaccine will be transferred.
- Fill in the Phone number for the transfer facility.
- Indicate whether a generator is available by checking the box for Yes or No.
- Write the Contact Name for the transfer facility.
- Input the Date of agreement.
- Specify where to obtain the necessary supplies.
- List a Phone number for the supply source.
- Indicate whether you will use Ice, Dry ice, or Cooler for transport.
- Provide details of the Shipping Agent including their Phone number and Tracking number.
- Document the name of the person who made contact with the LHD/HSR prior to transport.
- Record the Temperature of the refrigerator prior to transport.
- Ensure the Inventory of vaccine is done using the C-33 form, keeping a copy for your records.
- Label the bag with your PIN, clinic name, clinic contact, and phone number.
- Specify the container used to transport the refrigerated vaccine.
- Ensure ice packs are within the container and separated from vaccines using crumpled paper.
- Place a thermometer in the container.
- Document the Time and temperature in the container prior to transport.
- Identify the person transporting vaccine.
- Repeat steps for the transport of frozen vaccines, ensuring the Temperature of the freezer prior to transport is noted.
- Complete the Inventory of vaccine for frozen vaccines and follow similar labeling and documentation steps as above.
- In the event of a city-wide evacuation, fill in HSR Contact Name and their Phone number.
After completing the form, review it for accuracy and completeness. This ensures all necessary information is readily available for proper vaccine storage and transfer. It's essential to keep a copy of this form for your records.
What You Should Know About This Form
What is the purpose of the Contingency Plan For Vaccine Storage form?
This form is designed to guide healthcare facilities in preparing for unexpected situations that may disrupt the storage of vaccines. By outlining procedures for transferring and safeguarding vaccines, the plan ensures that vaccines remain effective and safe for patients. It serves as a proactive measure to prevent loss due to power outages, equipment failure, or emergencies.
Who is responsible for transferring the vaccines according to the form?
The form requires the designation of clinic staff responsible for the transfer of vaccines. Two names should be provided: one primary staff member and one back-up. This structure guarantees that there is always someone accountable for the safe and timely handling of vaccines in case of any unforeseen events.
What information is required about the facility in the form?
Facilities must fill out key details, including the facility name, TVFC PIN, address, city, state, zip code, and contact phone number. This information is crucial for tracking and communication, ensuring that the vaccines can be located and managed appropriately during transport or in an emergency.
What steps should be taken before transporting the vaccines?
Before transportation, several important steps must be completed. These include verifying the temperature of the refrigerator or freezer, preparing an inventory of vaccines using a specific form (C-33), and labeling bags with relevant information such as the PIN and clinic name. Correct preparation helps maintain proper temperatures and ensures that the vaccines are properly documented and handled.
How is the safety of the vaccines ensured during transport?
To ensure safety during transport, specific packaging procedures must be followed. For refrigerated vaccines, ice packs should be placed separately from the vaccines using crumpled paper for insulation. For frozen vaccines, dry ice is used in the transport container. Additionally, a thermometer should be included to monitor temperature throughout the journey. Proper documentation of time and temperature records is essential for accountability.
What should be done in case of a city-wide evacuation?
In the event of a city-wide evacuation, it is vital to contact your health service region for guidance on the evacuation plan. The form requires you to list the health service region contact name and phone number. This ensures you are informed of the procedures to follow, ultimately protecting the vaccines and your community's health.
Common mistakes
Filling out the Contingency Plan For Vaccine Storage form may seem straightforward, but several common mistakes can hinder the effectiveness of the document. Each error can have significant implications, particularly concerning vaccine safety and compliance standards. Recognizing these mistakes is essential for maintaining the integrity of vaccine storage during emergencies.
One of the most prevalent mistakes is failing to include accurate contact information. When listing the clinic's phone number or the names of responsible staff members, it is crucial to ensure that the details are correct and up to date. An incorrect phone number could lead to delays in reaching the designated individuals during emergencies, potentially jeopardizing vaccine integrity.
Another frequent oversight is neglecting to document emergency contact protocols. The form requires information about who to contact within the health service region during a crisis. Omitting this information or providing incomplete details can lead to confusion, especially when rapid decisions are required. Accurate documentation ensures a clear line of communication in high-pressure situations.
Individuals often forget to specify the container used for transport. This section is vital, as it outlines the specifics of how vaccines will be moved safely. Without details on the type of container, the risk of temperature fluctuations increases, which can diminish the effectiveness of the vaccines. Choosing the right transport method is critical to preserving vaccine viability.
Failure to record the temperature readings prior to transport is another common mistake. Documenting the temperature of the refrigeration units before transferring vaccines is essential for verifying that the vaccines have remained at the proper temperatures. This oversight may result in disputes regarding the vaccines' condition during transport.
In addition, incomplete inventory documentation is frequently seen on this form. It is vital that the inventory of vaccines is not only noted but that a copy is kept for future reference. Missing inventory records can complicate audits and prevent efficient tracking of vaccine supplies, making accountability more challenging.
Some individuals also neglect to label bags appropriately. All bags must clearly indicate the PIN, clinic name, clinic contact, and phone number. An unlabeled or improperly labeled bag can cause mix-ups and delays in retrieving the vaccines when they arrive at their destination. Accurate labeling simplifies identification and maintains organization.
Failing to specify the time and temperature in the container prior to transport can also lead to complications. This information is crucial for understanding the conditions the vaccines were exposed to before departure. Inaccuracies can undermine the reliability of the entire transport process.
Lastly, not having a well-prepared evacuation plan outlined in the form can pose serious risks during a city-wide emergency. Contacting the health service region in advance is paramount. Without a solid plan, clinics may face chaos when dealing with the transport of vaccines, which can lead to devastating consequences for public health.
Documents used along the form
When managing the logistics of vaccine storage and transportation, several supporting documents play a crucial role in ensuring a smooth process. Each form serves a distinct purpose and can help maintain safety and compliance during vaccine handling. Below is a summary of key forms that complement the Contingency Plan for Vaccine Storage form.
- Inventory List (C-33): This document tracks the types and quantities of vaccines on hand. It helps clinics ensure they have adequate supplies and provides essential information for transport.
- Temperature Log: A record that tracks the temperatures of storage units over time. Keeping an accurate log supports compliance with regulations and ensures vaccines are stored properly.
- Transfer Agreement: This document outlines the terms under which vaccines are transported between facilities. It confirms responsibilities and expectations, creating clarity for all parties involved.
- Facility Contact Sheet: A list of key contacts at both the sending and receiving facilities. This sheet provides quick access to contact information and emergencies, enhancing communication during transport.
- Shipping Instructions: A detailed set of protocols for securely packaging and shipping vaccines. Following these guidelines helps mitigate risks associated with transportation.
- Evacuation Plan: Created by local health services, this plan outlines procedures to follow during a city-wide evacuation. It ensures that vaccine safety is prioritized in emergencies.
- Thermometer Calibration Log: This document records the calibration dates of thermometers used during storage and transport. Ensuring accuracy in temperature monitoring is vital for vaccine integrity.
- Emergency Contact List: A comprehensive list of contacts for emergency situations, including state health officials and local health representatives. This resource is invaluable during unexpected events during transport.
Utilizing these documents in conjunction with the Contingency Plan for Vaccine Storage form enhances preparedness and risk management for vaccine handling. Effective coordination among staff and clear documentation can make a significant difference in public health outcomes.
Similar forms
-
Emergency Preparedness Plan: This document outlines procedures for various emergency scenarios within a healthcare setting. Like the Contingency Plan for Vaccine Storage, it emphasizes immediate actions to ensure safety and compliance, detailing who is responsible and what steps to take during a crisis.
-
Vaccine Storage and Handling Guidelines: This set of guidelines provides best practices for storage conditions and handling of vaccines. Similar to the Contingency Plan, it specifies temperature requirements and includes checklists to track compliance and ensure the efficacy of vaccines during transport.
-
Transport Policy Documentation: This document contains policies for the safe transportation of medical supplies, including vaccines. It parallels the Contingency Plan by outlining the roles of responsible personnel, transportation methods, and maintaining records of temperature and inventory throughout the transit process.
-
Disaster Recovery Plan: This plan elaborates on strategies to recover systems and processes after a disastrous event. It is similar as it serves to protect healthcare services and patient health by detailing lines of communication and responsibilities during emergencies affecting storage and distribution of vaccines.
-
Inventory Management Procedures: This document provides a framework for tracking medical supplies, including vaccines. Like the Contingency Plan, it emphasizes the importance of accurate inventory records to ensure that vaccine availability is maintained, especially during transport and emergencies.
Dos and Don'ts
When filling out the Contingency Plan For Vaccine Storage form, consider the following recommendations:
- Clearly specify the facility name and TVFC PIN to ensure proper identification.
- Provide accurate contact information for all staff involved in vaccine transfer.
- Document the temperature of both the refrigerator and freezer prior to transport.
- Include a detailed inventory of vaccines using the C-33 form and keep a copy for your records.
- Use ice packs or dry ice appropriately and separate them from the vaccines during transport.
Equally important are the actions to avoid:
- Do not skip any fields in the form; incomplete information may delay response efforts.
- Avoid using unapproved transportation methods or containers, as this can jeopardize vaccine integrity.
- Do not forget to record the time and temperature in the container before transport.
- Never neglect to make contact with your Local Health Department or Health Service Region prior to transport.
Misconceptions
When it comes to the Contingency Plan For Vaccine Storage form, many misconceptions can lead to confusion. Understanding what this form truly involves is essential. Here are ten common misconceptions:
- It's just paperwork. Many believe this form is only a formality. In reality, it's a critical guide for ensuring vaccine safety during emergencies.
- The plan is the same for all vaccines. Different vaccines require different storage conditions. This plan must be tailored to specific vaccines to maintain their effectiveness.
- Only one person needs to be responsible for transport. It is important to have a backup person designated. Emergencies can happen, and this ensures continuity in care.
- Temperature checks are optional. Proper temperature checks are essential. These checks confirm that the vaccines remain within safe limits during transport.
- Ice packs are the only way to maintain temperature. Depending on the vaccine type, dry ice and coolers can also be effective. The right container will protect the vaccines best.
- Contact with local health departments is not necessary. In fact, reaching out to local health departments before transport is crucial. They may provide guidance and support.
- The plan is unnecessary if the facility has good refrigeration. Even with proper refrigeration, unforeseen circumstances can occur. A contingency plan ensures readiness for all scenarios.
- Once the form is filled out, it’s done. The plan should be reviewed regularly. Keeping the plan updated is vital for an effective response during an emergency.
- There’s no need to document transport conditions. Documenting the time and temperature during transport is crucial. It provides vital information regarding the viability of the vaccines.
- Everyone automatically knows what to do in an emergency. Clear instructions in the form ensure that all staff are prepared. Miscommunication can lead to mishandling.
By addressing these misconceptions, individuals can better understand the importance of the Contingency Plan For Vaccine Storage form. This understanding can lead to better practices and ultimately safeguard public health.
Key takeaways
The following are key takeaways regarding the completion and utilization of the Contingency Plan For Vaccine Storage form:
- Facility Information: Complete the facility name, TVFC PIN, address, date, city, state, and zip code accurately to ensure correct identification.
- Contact Information: Include a phone number for the facility and the clinic staff responsible for the transfer of vaccines, ensuring all personnel are reachable.
- Backup Personnel: Designate a backup person for vaccine transfer to ensure continuity in case the primary contact is unavailable.
- Transfer Details: Clearly list the name and phone number of the facility to which vaccines will be transferred.
- Equipment Needs: Indicate whether a generator is available. This can help maintain temperature control during a power outage.
- Inventory Documentation: Use the C-33 form to record vaccine inventory, keeping a copy for personal records during transfers.
- Transport Containers: Always use appropriate containers that separate ice packs from vaccines to prevent damage.
- Temperature Monitoring: Document the temperature of both the refrigerator and freezer before transport to ensure vaccine integrity.
- Labeling: All bags should be labeled properly with the clinic's PIN, name, and contact information for easy identification during transport.
- Emergency Procedures: In case of a city-wide evacuation, it is critical to have the HSR contact name and phone number ready for access to evacuation plans.
Browse Other Templates
Detroit Tigers Donation Request - All requests should express a genuine intent to benefit the local community.
Export Value Declaration Form - Exporters must include their United States Employer Identification Number (EIN) if applicable.