Fill Out Your Controlled Drug Log Form
In the realm of healthcare and pharmacy management, the Controlled Drug Log form plays a pivotal role in ensuring the safe and compliant handling of controlled substances. Designed to maintain meticulous records, this form tracks essential information such as the type of controlled substance, its strength, and size, fostering accountability among healthcare professionals. Each entry includes critical details like the date of receipt, the distributor’s invoice number, and the amount received, enabling organizations to maintain accurate inventory levels. Additionally, the log captures the names or initials of staff members who have removed any quantities, promoting a culture of transparency and responsibility. By adhering to the guidelines set forth in this log, healthcare facilities not only comply with regulatory requirements but also safeguard against misuse or diversion of these potent medications, ultimately prioritizing patient safety in every transaction.
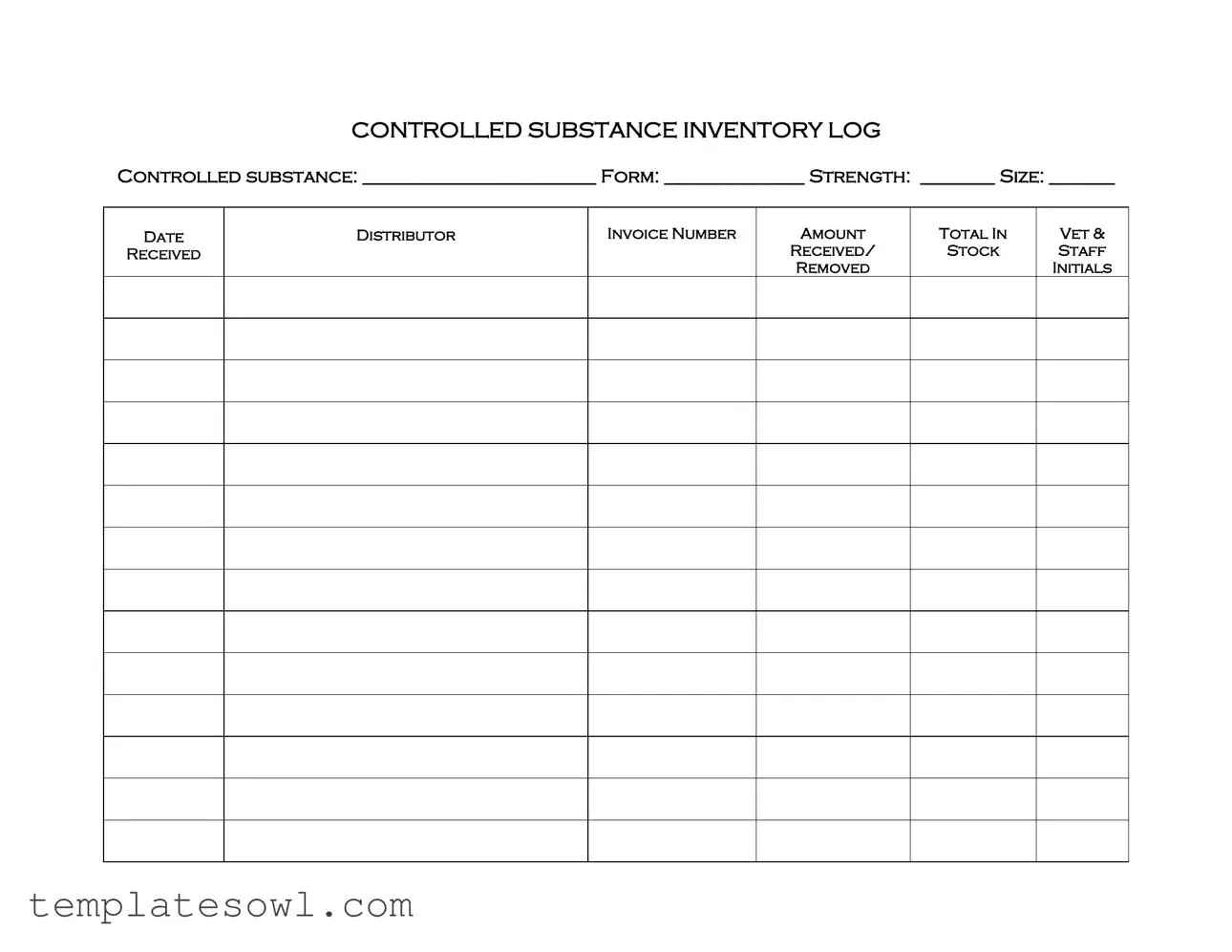
Controlled Drug Log Example
CONTROLLED SUBSTANCE INVENTORY LOG
CONTROLLED SUBSTANCE: _________________________ FORM: _______________ STRENGTH: ________ SIZE: _______
DATE |
DISTRIBUTOR |
INVOICE NUMBER |
AMOUNT |
TOTAL IN |
VET & |
RECEIVED |
|
|
RECEIVED/ |
STOCK |
STAFF |
|
|
|
|
|
|
|
|
|
REMOVED |
|
INITIALS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Form Characteristics
| Fact Name | Description |
|---|---|
| Purpose | The Controlled Drug Log is used to track the inventory of controlled substances. It ensures compliance with legal regulations regarding their use and distribution. |
| Required Information | The form requires essential details: controlled substance name, form, strength, size, date, distributor, invoice number, amount, and staff initials. |
| Legal Requirement | In many states, maintaining a controlled substance log is mandated by law to prevent misuse and ensure proper handling of these drugs. |
| Storage and Accessibility | Logs should be securely stored but remain accessible to authorized staff for frequent updates and audits. |
| Frequency of Updates | It is crucial to update the Controlled Drug Log regularly, ideally after each transaction that involves the receipt or removal of a controlled substance. |
| Governing Laws | The specific laws governing the use of the Controlled Drug Log vary by state. For example, California mandates strict record-keeping under the Business and Professions Code Section 4160. |
| Importance of Accuracy | Accurate record-keeping helps identify discrepancies and can protect against legal penalties. Carelessness in records can lead to audits or fines. |
Guidelines on Utilizing Controlled Drug Log
In order to maintain compliance with controlled substance regulations, the Controlled Drug Log form must be filled out accurately. This ensures all pertinent information regarding controlled substances is documented systematically and can be referenced as needed.
- Begin by entering the controlled substance name in the designated space at the top of the form.
- Next, fill in the form that corresponds to the substance indicated.
- Provide the strength of the controlled substance in the specified area.
- Record the size of the controlled substance container, such as the count or volume.
- In the appropriate columns, fill in the date when the controlled substance was received.
- Document the distributor name responsible for supplying the controlled substance.
- Enter the invoice number that pertains to the transaction for record-keeping purposes.
- Log the amount of the controlled substance received.
- Indicate the total amount in the designated field based on what was received.
- Have the staff initials of the person responsible for receiving the substance entered in the provided space.
- Finally, ensure that the section for any removals from stock is filled out properly, including the initials of the staff member who removed the substance.
What You Should Know About This Form
What is the purpose of the Controlled Drug Log form?
The Controlled Drug Log form helps track the use and distribution of controlled substances within a facility. This log is essential for maintaining accurate records and ensuring compliance with regulations. It helps to monitor inventory levels and prevent misuse or diversion of these substances.
What information do I need to fill out on the form?
You'll need to provide several key details on the form. These include the controlled substance's name, form (e.g., tablet, liquid), strength, size, date of receipt, name of the distributor, invoice number, quantity received, and the initials of the staff member who logged it. This information provides a complete picture of the drug’s status in the facility.
How should I record the amount of controlled substance received?
When you receive a controlled substance, you should note the exact amount in the designated space on the form. Ensure that you verify the quantity against the invoice before recording it. Keeping an accurate amount prevents discrepancies and helps keep records in order.
Who is responsible for filling out the Controlled Drug Log form?
Typically, the staff member who receives the controlled substance is responsible for filling out the log. However, it’s good practice to have a designated person or team oversee the log to ensure consistency and compliance with regulations.
What should I do if I notice an error on the form?
If you find an error on the Controlled Drug Log form, you should correct it immediately. Cross out the incorrect information and write the correct details next to it, then initial the change. This practice is important for maintaining accurate records and demonstrating accountability.
How often should the Controlled Drug Log be reviewed?
It’s advisable to review the Controlled Drug Log regularly, ideally after each transaction or at least weekly. Regular reviews help in identifying inconsistencies, ensuring compliance, and managing inventory effectively.
What should I do if a controlled substance is lost or stolen?
In case of loss or theft of a controlled substance, it’s crucial to report the incident immediately to your supervisor. An investigation should be initiated, and proper documentation should be completed. This step helps in tracking issues and improving security measures.
Can I use the Controlled Drug Log form for substances that are not controlled?
No, the Controlled Drug Log form is specifically designed for controlled substances. Using it for non-controlled items may lead to confusion and improper record-keeping. It's better to use a different log or inventory management system for non-controlled substances.
Are there any specific storage requirements for controlled substances?
Yes, controlled substances must be stored securely. They should be kept in a locked cabinet or safe, with limited access to authorized personnel only. Adhering to these guidelines not only ensures safety but also complies with legal requirements.
Common mistakes
When filling out the Controlled Drug Log form, it’s not uncommon for individuals to make mistakes that could lead to significant issues down the line. Understanding these common pitfalls is crucial for maintaining compliance and ensuring proper inventory management. Here are ten frequent mistakes to avoid.
First, people often leave the Controlled Substance field blank. This can result in confusion regarding what exactly is being logged. It is essential to clearly specify the substance, as this ultimately helps in tracking and accountability.
A second mistake frequently encountered is failing to complete all sections of the form properly. Each field, from FORM to AMOUNT and TOTAL RECEIVED, must be filled out diligently. Incomplete forms can lead to discrepancies, making audits more challenging.
Many also overlook the importance of the DATE RECEIVED. Without documenting the date, it becomes nearly impossible to monitor when substances are received and how they are utilized. This oversight can confuse inventory audits and impact compliance with regulations.
Distributor Invoice Numbers are another area where mistakes are common. Failing to include this information can create a lack of traceability. Each controlled substance should have a corresponding invoice number to corroborate its origin and support any audits.
Some individuals mistakenly record the AMOUNT RECEIVED incorrectly, often due to a simple transcription error. Double-checking these figures can spare you from operational headaches later. Accurate amounts are vital for inventory control and regulatory compliance.
Moreover, it's important to ensure that staff initials are present, and corresponding entries are signed by the right personnel. Missing initials can lead to accountability issues when trying to trace who handled specific transactions or removals.
Another common mistake involves not keeping the log updated in real time. Lagging behind can result in inaccuracies that compromise both your inventory and compliance status. Regular updates ensure that the log reflects actual usage and stock levels.
People also often forget to note the STRENGTH and SIZE of the controlled substance. These details matter; they ensure correct dosages are administered and that the log aligns with prescriptions issued.
Furthermore, some individuals neglect to properly document the TOTAL IN STOCK after substances have been removed. This error leads to an inflated perception of available drugs, which can have serious repercussions if a legitimate need arises.
Finally, maintaining a consistent format is essential. Inconsistent entries make it difficult to track down data quickly. Whether it’s the spacing in dates or the manner of writing entries, consistency fosters clarity, enhancing the usability of the log.
By being aware of and avoiding these ten common mistakes, individuals who handle controlled substances can contribute significantly to the safety and compliance of their practices. Diligent attention to detail is not just encouraged; it is necessary for everyone's well-being.
Documents used along the form
In managing controlled substances, specific forms and documents are essential to ensure compliance and maintain accurate records. Here is a list of other documents often used alongside the Controlled Drug Log form.
- Controlled Substance Inventory Log: This document tracks the total amount of controlled substances on hand. It provides a snapshot of inventory levels and helps in reconciling discrepancies during audits.
- Order Forms: These forms are used to request additional controlled substances from suppliers. They include details such as the type, quantity, and purpose of the substances being ordered.
- Receiving Reports: This document confirms the arrival of controlled substances. It includes information about the shipment, ensuring that the received quantities match the ordered amounts.
- Dispensing Records: These records outline the distribution of controlled substances to patients or within the facility. They include patient details and amounts dispensed to track usage accurately.
- Transfer Forms: Used when controlled substances are moved between departments or locations, these forms document the specifics of the transfer to maintain accountability.
- Destruction Log: This log records the disposal of expired or unused controlled substances. It ensures compliance with regulations governing the safe disposal of these materials.
- Audit Reports: These reports summarize the findings of routine inspections. They assess adherence to protocols and identify potential issues in managing controlled substances.
- Staff Training Records: These documents track training sessions completed by staff on handling and managing controlled substances, ensuring everyone involved understands the protocols and regulations.
Proper documentation is crucial for the effective management of controlled substances. Utilizing these forms helps maintain compliance, accountability, and safety within any facility handling these sensitive materials.
Similar forms
The Controlled Drug Log form plays a crucial role in tracking and managing controlled substances. Similar documentation helps in maintaining regulatory compliance and ensuring accountability in handling these drugs. Below are four documents that share similarities with the Controlled Drug Log form:
- Controlled Substance Inventory Log: This document serves to maintain a comprehensive record of all controlled substances in inventory. Like the Controlled Drug Log, it requires detailed information regarding the type of controlled substance, its strength, and quantities, ensuring that all items are accounted for properly.
- Prescription Record: This document tracks the prescriptions written for controlled substances. Similar to the Controlled Drug Log, it includes essential details such as the patient’s name, the drug prescribed, dosage, and the prescribing physician’s information, ensuring safe and legitimate usage of controlled drugs.
- Dispensing Log: This log records the dispensing of controlled substances to patients or facilities. It resembles the Controlled Drug Log by capturing critical data such as the date, amount dispensed, and the initials of the dispensing staff, drawing parallels in the accountability of controlled substances.
- Receipt Log: This documentation reflects the receipt of controlled substances from vendors. It aligns with the Controlled Drug Log as it includes core elements like the date, invoice number, and amount received, thereby promoting a transparent audit trail for inventory management.
Dos and Don'ts
Filling out a Controlled Drug Log form is an important process in managing controlled substances responsibly. To ensure compliance and accuracy, follow these guidelines:
- DO: Fill in all required fields completely.
- DO: Use clear, legible handwriting or type the information.
- DO: Double-check amounts to ensure they match the invoice.
- DO: Initial all entries to provide accountability.
- DO: Keep the log in a secure, accessible location.
- DO: Report any discrepancies immediately.
- DON'T: Leave any fields blank unless specified.
- DON'T: Use correction fluid or erase entries.
- DON'T: Rely on memory; always refer to the invoice.
- DON'T: Allow unauthorized persons access to the log.
- DON'T: Forget to update the log regularly.
- DON'T: Store the log in an unmonitored or public area.
Misconceptions
The Controlled Drug Log form plays a crucial role in the management of controlled substances. However, various misconceptions surround its use. Clarifying these misunderstandings is essential for ensuring compliance and proper storage of medications.
- Misconception 1: The log only needs to be filled out when drugs are received.
- Misconception 2: Only one person can complete the log.
- Misconception 3: Any type of drug can be recorded on the log.
- Misconception 4: Errors in the log can be crossed out or erased.
- Misconception 5: The log can be kept in any location.
- Misconception 6: Only veterinarians are responsible for the log.
- Misconception 7: It is acceptable to skip days when no drugs are used.
- Misconception 8: The log is only required during inspections.
- Misconception 9: A digital version of the log is sufficient to meet requirements.
This is incorrect. The log must also document when drugs are administered or removed from stock. This ensures accountability throughout the entire process.
In fact, multiple staff members may contribute to the log. However, all entries must be clear and signed off by authorized personnel to maintain integrity.
The Controlled Drug Log is specifically designed for controlled substances. Other medications should not be logged here.
Controlled Drug Logs must be stored securely. Keeping them in a locked area is crucial to prevent unauthorized access to sensitive information.
All staff handling controlled substances share the responsibility for accurate recordkeeping. Accountability lies with the entire team.
Even on days when no controlled substances are administered, it is important to maintain records. Consistency in documentation is vital for compliance.
Routine maintenance of the log is necessary, regardless of whether an inspection is imminent. Keeping accurate and timely records benefits everyone.
While electronic logs can be beneficial, they must comply with specific regulatory standards. Paper logs may still be required depending on local laws and facility policies.
Understanding these misconceptions allows for better compliance and ultimately safer handling of controlled substances.
Key takeaways
When filling out and using the Controlled Drug Log form, consider the following key takeaways:
- Always fill in the controlled substance name clearly to avoid confusion.
- Check that you include the correct form, strength, and size of the controlled substance.
- Document the date received to maintain an accurate record.
- Make sure to include the distributor's name for tracking purposes.
- Record the invoice number as it helps with accountability.
- Note the amount received and the total stock on hand.
- Ensure that staff initials are included when a substance is removed from stock.
- Regularly review the log for accuracy and completeness to ensure compliance.
Browse Other Templates
New York State Certificate of Authority - Applicants must provide contact information, including telephone and fax numbers.
Fax Filing Document,Court Communication Form,Superior Court Transmission Sheet,Attorney Correspondence Cover Sheet,Legal Fax Submission Form,Court Document Transmission Cover,Fax Notice for Court Filing,Judicial Fax Cover Page,California Court Fax Fo - Understanding how to properly use this form is essential for effective representation.