Fill Out Your Drug Screen Form
The Drug Screen form is an essential document in the realm of workplace safety and compliance, particularly when it comes to drug testing. This form is designed to collect and maintain crucial information during the drug testing process, ensuring that every step is carried out according to established regulations. From identifying the employer and the donor to specifying the testing authority—be it HHS, NRC, or DOT—the form categorically lays out the necessary details. It also includes the reasons for testing, which might range from pre-employment screenings to post-accident evaluations. The form accommodates a variety of drug tests, including those for common substances like THC, cocaine, and amphetamines, allowing employers to tailor their screening process as needed. In addition, a structured chain of custody is established, ensuring that the specimen is collected, labeled, sealed, and logged systematically, thus safeguarding the integrity of the testing process. With specific sections devoted to the collector’s remarks, temperature readings, and the condition of the specimen, the Drug Screen form maintains a rigorous approach to accountability and transparency. Finally, at each stage of the process, signatures from both collectors and certifying scientists affirm the authenticity of the results, underscoring the importance of following protocols closely to uphold workplace safety and legal standards.
Drug Screen Example
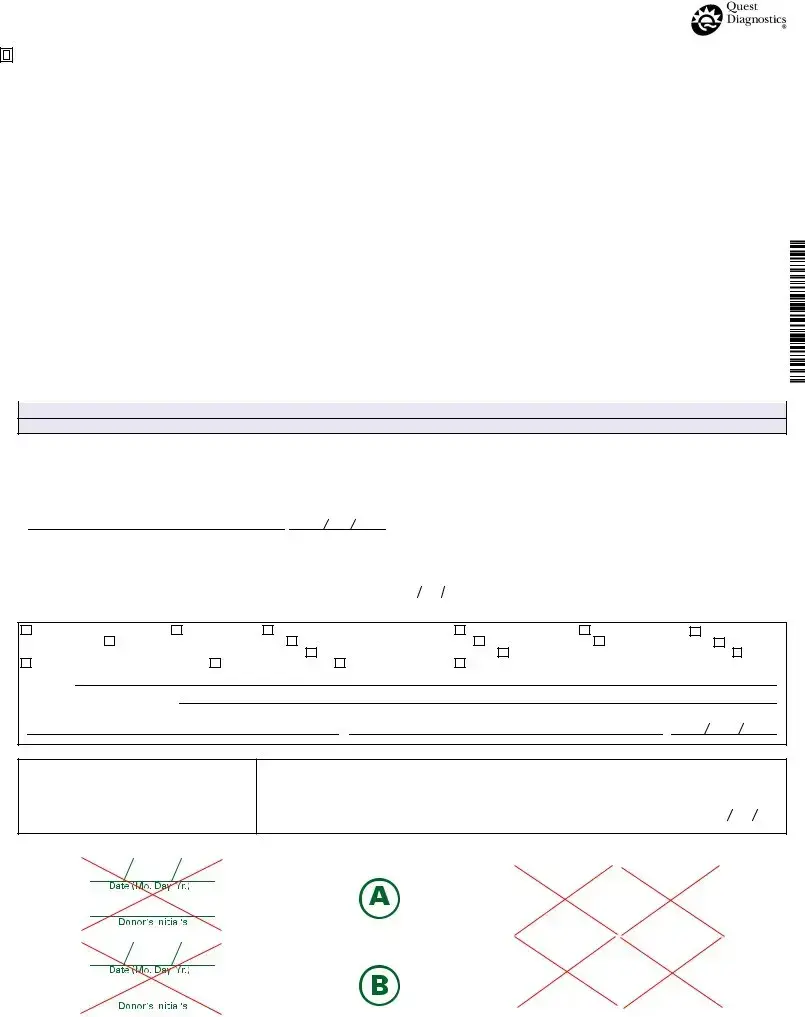
FEDERAL DRUG TESTING CUSTODY AND CONTROL FORM
SPECIMEN ID NO. |
|
STEP 1: COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE |
LAB ACCESSION NO. |
Quest, Quest Diagnostics, the associated logo and all associated Quest Diagnostics marks are the trademarks of Quest Diagnostics Incorporated. © Quest Diagnostics Incorporated. All rights reserved.
A. Employer Name, Address, I.D. No. |
|
|
B. MRO Name, Address, Phone and Fax No. |
||||||||||
|
|
|
|
|
|
|
|
|
|||||
C. Donor SSN or Employee I.D. No. _______________________________________________________________ |
|
|
|
|
|||||||||
D. SpecifyTesting Authority: HHS |
NRC |
DOT – Specify DOT Agency: FMCSA |
FAA |
FRA FTA PHMSA USCG |
|||||||||
E. Reason forTest: |
Random |
Reasonable Suspicion Cause Post Accident |
Return to Duty |
|
|||||||||
F. DrugTests to be Performed: |
THC, COC, PCP, OPI, AMP |
THC & COC Only |
Other (specify) ________________________________________________ |
||||||||||
G. Collection Site Name: |
|
|
|
|
|
Collection Site Code: |
|
|
|
|
|||
Address: |
|
|
|
|
|
|
Collector Phone No.: |
|
|
||||
City, State and Zip: |
|
|
|
|
|
Collector Fax No.: |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STEP 2: COMPLETED BY COLLECTOR (make remarks when appropriate) Collector reads specimen temperature within 4 minutes.
Temperature between 90° and 100° F? Yes No, Enter Remark |
Collection: Split Single None Provided, Enter Remark |
Observed, (Enter Remark) |
REMARKS
STEP 3: Collector affixes bottle seal(s) to bottle(s). Collector dates seal(s). Donor initials seal(s). Donor completes STEP 5 on Copy 2 (MRO Copy)
STEP 4: CHAIN OF CUSTODY - INITIATED BY COLLECTOR AND COMPLETED BY TEST FACILITY
|
I certify that the specimen given to me by the donor identified in the certification section on Copy 2 of this form was |
|
SPECIMEN BOTTLE(S) RELEASED TO: |
|||||||
|
collected, labeled, sealed, and released to the Delivery Service noted in accordance with applicable Federal requirements. |
Quest Diagnostics Courier |
||||||||
|
|
X |
|
|
|
|
|
FedEx |
||
|
|
Signature of Collector |
|
|
|
|
|
Other |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AM |
|
|
|
|
|
|
|
|
|
|
PM |
|
|
|
|
|
|
(Print) Collector's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|
Time of Collection |
|
|
Name of Delivery Service |
||
RECEIVED AT LAB OR IITF: |
|
|
|
|
|
Primary Specimen |
SPECIMEN BOTTLE(S) RELEASED TO: |
|||
|
X |
|
|
|
|
|
Bottle Seal Intact |
|
||
|
|
|
|
|
|
Yes No |
|
|||
|
|
Signature of Accessioner |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If No, Enter remarks |
|
|
|
|
|
|
|
|
|
|
in Step 5A. |
|
|
|
|
(Print) Accessioner’s Name (First, MI, Last) |
|
|
|
Date (Mo./Day/Yr.) |
|
|||
STEP 5A: PRIMARY SPECIMEN REPORT - COMPLETED BY TEST FACILITY
NEGATIVE |
POSITIVE for: |
Marijuana Metabolite ( |
6- Acetylmorphine |
Methamphetamine |
MDMA |
|
DILUTE |
|
|
Cocaine Metabolite (BZE) |
Morphine |
Amphetamine |
MDA |
|
|
|
PCP |
Codeine |
|
MDEA |
REJECTED FOR TESTING |
ADULTERATED |
SUBSTITUTED |
INVALID RESULT |
|
|
|
REMARKS:
Test Facility (if different from above):
I certify that the specimen identified on this form was examined upon receipt, handled using chain of custody procedures, analyzed, and reported in accordance with applicable Federal requirements.
X
Signature of Certifying Scientist |
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
STEP 5b: COMPLETED BY SPLIT TESTING LABORATORY
RECONFIRMED FAILED TO RECONFIRM - REASON ____________________________________________
___________________________________________ |
I certify that the split specimen identified on this form was examined upon receipt, handled using chain of custody |
||||||||||||||||||||||||||||
procedures, analyzed and reported in accordance with applicable Federal requirements. |
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Name |
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
___________________________________________ |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Signature of Certifying Scientist |
|
|
|
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Address |
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMB No.
PRESS HARD - YOU ARE MAKING MULTIPLE COPIES
Form Characteristics
| Fact Name | Details |
|---|---|
| Form Purpose | The Drug Screen form is used to document the collection and testing of urine samples for drug screening according to federal regulations. |
| Key Sections | The form includes sections for employer and collector information, as well as testing authority, reason for the test, and collection site details. |
| Compliance Requirement | This form must be filled out in compliance with federal laws that govern drug testing, including requirements from the Department of Transportation (DOT) and the Health and Human Services (HHS). |
| Chain of Custody | Maintaining the chain of custody is crucial. The collector must properly label and seal the specimen to ensure integrity during transport. |
| Result Reporting | Testing facilities must report results following the completion of the specimen analysis, ensuring compliance with federal guidelines. |
Guidelines on Utilizing Drug Screen
Once you have the Drug Screen form in hand, you need to complete it carefully to ensure all necessary information is accurately recorded. Filling out this form is crucial as it sets the stage for the testing process that follows.
- Begin with the top section. The collector or employer representative fills out the Employer Name, Address, and I.D. No..
- Next, provide the MRO Name, Address, Phone, and Fax No..
- Enter the donor’s SSN or Employee I.D. No..
- Specify the Testing Authority by marking the relevant option (HHS, NRC, DOT), and indicate the DOT Agency if applicable.
- State the Reason for Test by selecting one of the provided options or writing in another reason, if necessary.
- List the Drug Tests to be Performed by selecting from the options given or specifying any other tests.
- Fill in the Collection Site Name, Code, Address, Phone No., City, State, and Zip, along with the Collector Fax No..
- Have the collector check the specimen temperature within 4 minutes and record whether it falls between 90° and 100° F.
- Indicate whether the collection was Split, Single, or None Provided and make remarks as needed.
- The collector affixes the bottle seal(s) to the specimen bottles, dates the seals, and has the donor initial the seals.
- The donor should complete Step 5 on Copy 2 (the MRO copy).
- Initiate the chain of custody by having the collector certify the specimen was collected, labeled, sealed, and released following federal requirements.
- Record the Delivery Service used and ensure the collector’s name, signature, and the date and time of collection are included.
- In Step 5A, the test facility completes the primary specimen report, indicating if results are negative, positive, or if the specimen was rejected for testing.
- Finally, if applicable, the split testing laboratory completes Step 5B, confirming whether the split sample was reconfirmed or not.
What You Should Know About This Form
What is the purpose of the Drug Screen form?
The Drug Screen form, formally known as the Federal Drug Testing Custody and Control Form, serves as a standardized procedure for collecting and handling urine specimens for drug testing. This form ensures that the testing process remains consistent and adheres to federal regulations. It documents the chain of custody and verifies that all the necessary steps are completed properly to eliminate chances for tampering or errors.
Who is responsible for completing the Drug Screen form?
The responsibility for completing the Drug Screen form primarily falls on the collector or the employer representative. They are tasked with accurately filling out each section, which includes details about the donor, testing authority, reason for the test, and the drugs being tested for. This detailed information is crucial for validity and compliance with federal requirements.
What types of drug tests can be performed according to the form?
The Drug Screen form allows for testing of various substances. Commonly tested drugs include THC (Marijuana), COC (Cocaine), PCP (Phencyclidine), OPI (Opiates), and AMP (Amphetamines). The form also provides options for specific combinations or for testing different drugs if specified. This flexibility ensures that testing can be tailored to meet specific employer policies or regulatory requirements.
How is the chain of custody maintained during the testing process?
Maintaining the chain of custody is vital for ensuring the integrity of the drug testing process. The collector initiates this by labeling and sealing the specimen bottles during collection. Each member of the testing process, from the collector to the laboratory, documents their handling of the specimen on the form. These records are crucial in establishing that the sample has not been tampered with and has been managed following federal guidelines throughout the entire testing process.
What happens after the specimen is collected?
Once the specimen is collected, the collector will seal the specimen bottles and fill out the form appropriately. The specimen is then sent to a designated laboratory for analysis. The laboratory performs the tests and provides results, which are documented on the Drug Screen form. If there are any issues, such as a positive or invalid result, the collection site will make further remarks on the form, ensuring a thorough record of the entire process.
Common mistakes
When filling out the Drug Screen form, several common mistakes can occur. Addressing these errors during the completion process can help ensure accurate results and compliance with federal regulations.
One mistake often made is misidentifying the testing authority. The form allows for specific designations such as HHS, NRC, and DOT. Failing to select the appropriate option can lead to confusion regarding the testing procedures and may invalidate the results. It is crucial to pay close attention to this section and ensure that the selected authority accurately reflects the reason for the test.
Another frequent error involves improperly filling out the donor’s identification section. This includes either the Social Security Number (SSN) or Employee ID Number. Omitting this information or providing incorrect details can create complications during the testing process. It can also lead to difficulties in tracing and managing records in the future. Always double-check these entries for accuracy before submission.
Additionally, participants sometimes forget to provide clear remarks in the collection section. The collector should note any irregularities, such as unusual specimen temperatures or collection methods. Ignoring this can lead to a lack of needed information for the testing facility, potentially resulting in disputes about the validity of the test. Collectors should ensure that relevant details are fully documented to maintain the integrity of the testing process.
Finally, a common oversight occurs when the chain of custody is not adequately maintained. This requires careful record-keeping as the specimen moves from the collector to the testing facility. Failing to document each step, sign, or date may create legal issues later. It is essential to understand that these procedures are in place to protect both the donor's rights and the integrity of the test results.
Documents used along the form
When conducting drug screening, several other documents often accompany the Drug Screen form. These forms help ensure proper tracking, compliance, and management of the testing process. Understanding each document's purpose can clarify your obligations and streamline the overall procedure.
- Chain of Custody Document: This form tracks the sample from collection to analysis, ensuring that it has not been tampered with. It provides a clear record of who handled the sample and when.
- Donor Consent Form: This document confirms that the donor understands the testing process and has agreed to provide a sample. It often outlines their rights and responsibilities.
- Medical Review Officer (MRO) Form: This form is completed by a medical review officer who checks the test results and manages any discrepancies, such as legitimate medical reasons for positive results.
- Specimen Collection Log: This log records all specimens collected over a specific time. It includes dates, times, and the names of donors, ensuring accurate tracking.
- Test Results Report: This document provides the outcome of the drug tests conducted. It details whether the results were negative or positive and lists specific substances tested.
- Follow-up Testing Form: If a donor requires additional testing, this form outlines the schedule and conditions for follow-up tests, ensuring compliance with procedures.
- Incident Report Form: If there is a reason to question the validity of a test (e.g., a spill or a mix-up), this form documents the incident for review and ensures corrective actions are followed.
Familiarity with these documents can help streamline the drug screening process. By ensuring that all necessary forms are completed and submitted, you can facilitate effective management and compliance throughout the testing protocol.
Similar forms
- Chain of Custody Form: Similar to the Drug Screen form, a Chain of Custody form documents who collected a sample, who handled it, and when it was analyzed. This form ensures the integrity of the specimen by establishing a clear record of its movement and custody throughout the testing process.
- Medical Release Form: Like the Drug Screen form, a Medical Release form authorizes healthcare providers to share an individual's health information. Both documents are essential in managing sensitive information while ensuring compliance with regulations such as HIPAA.
- Consent Form: A Consent form, which is often required for drug testing, is akin to the Drug Screen form in that it obtains permission from the donor for the testing process. Both documents protect the rights of individuals and confirm that they are aware of and agree to the procedures being conducted.
- Test Result Confirmation Form: This document serves a purpose similar to that of the Drug Screen form by summarizing the findings of a drug test. The Test Result Confirmation form validates the test outcomes and provides an official report, often required for record-keeping or for decision-making regarding employment or legal matters.
Dos and Don'ts
When completing the Drug Screen form, several important guidelines can help ensure accuracy and compliance. Below are six recommendations that highlight what to do and what to avoid.
- Do read the instructions thoroughly before starting.
- Do provide accurate and complete information in all fields.
- Do double-check the donor's identification details, such as the SSN or Employee ID.
- Do write clearly and legibly to avoid misinterpretation.
- Don't leave any mandatory fields blank.
- Don't forget to sign and date the form where required.
Misconceptions
Misconceptions about the Drug Screen form can lead to confusion regarding its purpose, process, and implications. Here are nine common misunderstandings:
- The form is only for pre-employment screening. Many people think this form is solely for potential employees. In reality, it is used for various reasons including random testing, post-accident evaluations, and return-to-duty testing.
- Drug testing is always mandatory. Although certain industries require drug testing, it is not universally compulsory. Employers may elect to test based on their policies or legal obligations.
- A negative test result guarantees employment. While a negative result may seem advantageous, various factors, such as other qualifications and the hiring process, ultimately influence employment decisions.
- The form guarantees privacy. Participants might assume that their test results are entirely confidential. However, there are circumstances under which employers and legal authorities may access this data, especially in regulated industries.
- Only illegal substances are tested. Some individuals believe that only illegal drugs are on the testing panel. However, legal substances, such as alcohol or prescription medications, can also be tested and reported if they affect job performance.
- Once the test is completed, there's no further responsibility. After the collection, the form may seem complete, but both the donor and the collector have ongoing responsibilities regarding the chain of custody and results communication.
- A positive result means instant termination. Many think that a positive test automatically results in job loss. However, most employers have a protocol that may include reviewing the results, offering counseling, or providing another chance.
- The form is the same across all companies. Although many aspects of the Drug Screen form are standardized, companies may have specific protocols or versions tailored to meet their unique requirements.
- All substances remain on the test for the same duration. People often believe that all drugs can be detected for an equivalent period. In fact, various substances have different detection windows based on numerous factors, including metabolism and frequency of use.
Understanding these misconceptions can lead to a more informed approach to drug screening processes and help demystify the Drug Screen form's intentions and functions.
Key takeaways
When it comes to filling out and using the Drug Screen form, understanding the following key points will ensure a smoother process and help maintain compliance with applicable regulations.
- Accurate Information: Make sure to provide complete and correct details in all sections. This includes employer and collector information, as well as the donor's identification.
- Testing Authority Selection: Clearly specify the testing authority from the options available, such as HHS, NRC, or DOT. Each has different guidelines that must be followed.
- Reason for Test: Indicate the reason for conducting the test. Options include pre-employment, random tests, and follow-up tests. This clarity is crucial for record-keeping.
- Drug Tests Specified: Be thorough when selecting which drug tests will be performed. Options like THC, COC, and PCP must be marked appropriately based on the testing requirements.
- Collector’s Role: The collector must properly document observations, including specimen temperature readings, to ensure validity. They are responsible for confirming the specimen’s integrity.
- Chain of Custody: Follow chain of custody protocols to maintain the integrity of the specimen. This process must be documented carefully at every step from collection to testing.
- Release of Specimen: Ensure that the specimen is handed over to the appropriate delivery service and that records reflect the time and manner of release.
- Test Results: After testing, actively confirm whether results are negative, positive, or rejected. Be aware of different classifications for test results, such as 'DILUTE' or 'INVALID.'
- Signature Requirements: Signatures from all parties involved, including the collector and certifying scientist, are essential for validation. Missing signatures can lead to complications later.
- Multiple Copies: Make use of the instruction "PRESS HARD - YOU ARE MAKING MULTIPLE COPIES" to ensure that all necessary parties have adequate documentation for their records.
By paying close attention to these elements, all involved parties can facilitate a more organized and legally compliant drug screening process.
Browse Other Templates
Opnav 5239/14 Fillable - It records user identification to verify individuals seeking access to sensitive information.
How to Make a Order Form - Utilize the additional note section for any custom instructions or notes.
