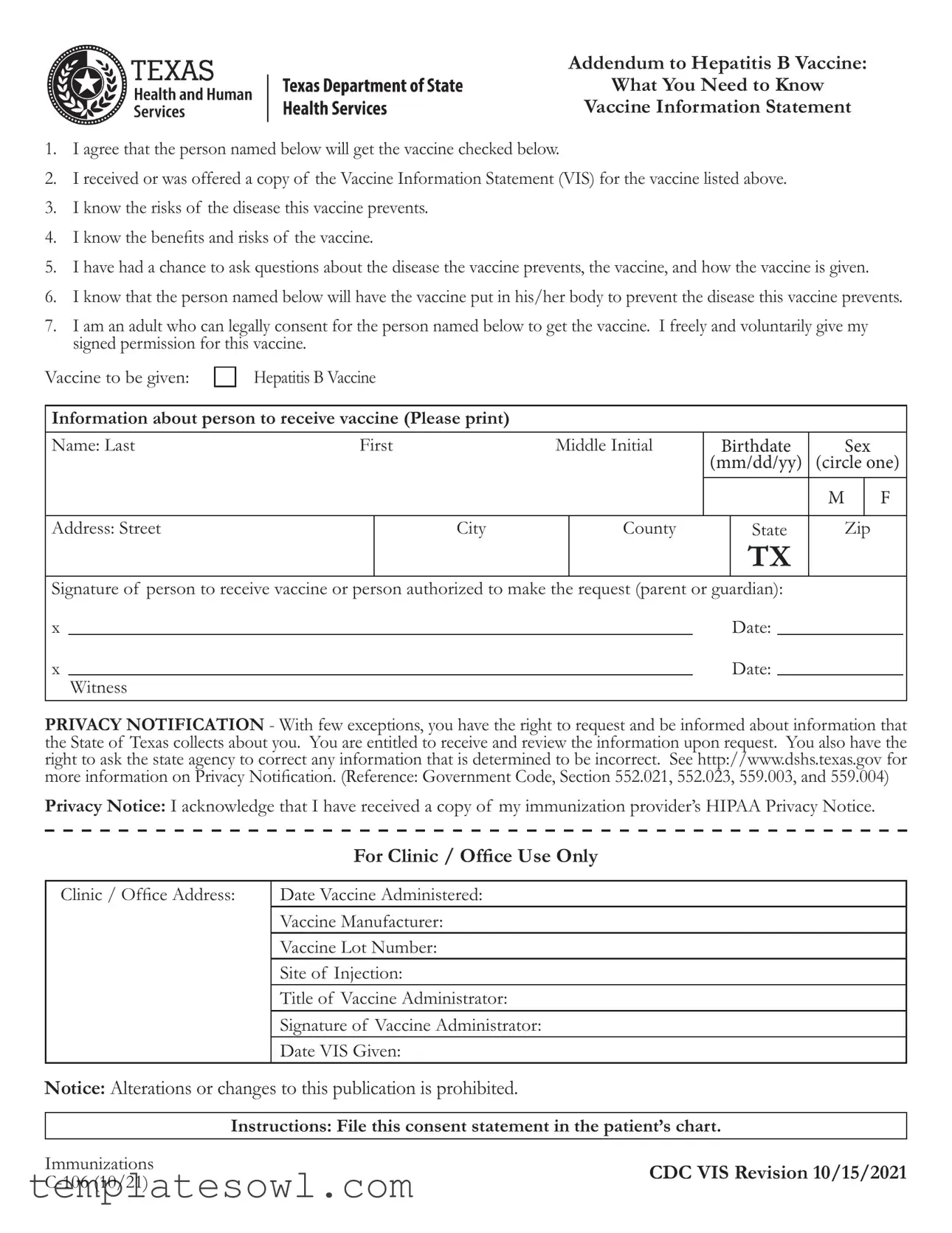
Fill Out Your Ec 106 Form
The EC 106 form serves as an essential consent document for individuals receiving the Hepatitis B vaccine, emphasizing informed decision-making in the vaccination process. It outlines the responsibilities of both the vaccine recipient and the individual providing consent, usually a parent or guardian, to ensure clarity and understanding before vaccination occurs. Key elements of the form include an acknowledgment of receipt of the Vaccine Information Statement (VIS), which contains critical information regarding the benefits and risks associated with the vaccine. It also highlights the opportunity for individuals to ask questions about both the disease and the vaccine itself, reinforcing the importance of informed consent. Moreover, the form captures personal information about the vaccine recipient, such as name and date of birth, and requires signatures to validate consent. Privacy protections are addressed, informing individuals of their rights to access and correct their data under state law. By carefully completing this form, individuals ensure that they are actively participating in their healthcare choices while also adhering to the necessary legal protocols associated with vaccine administration.
Ec 106 Example

Addendum to Hepatitis B Vaccine:
What You Need to Know
Vaccine Information Statement
1.I agree that the person named below will get the vaccine checked below.
2.I received or was offered a copy of the Vaccine Information Statement (VIS) for the vaccine listed above.
3.I know the risks of the disease this vaccine prevents.
4.I know the benefits and risks of the vaccine.
5.I have had a chance to ask questions about the disease the vaccine prevents, the vaccine, and how the vaccine is given.
6.I know that the person named below will have the vaccine put in his/her body to prevent the disease this vaccine prevents.
7.I am an adult who can legally consent for the person named below to get the vaccine. I freely and voluntarily give my signed permission for this vaccine.
Vaccine to be given: |
Hepatitis B Vaccine |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Information about person to receive vaccine (Please print) |
|
|
|
|
|
|
|
|
|
|
|||
Name: Last |
First |
Middle Initial |
Birthdate |
|
Sex |
||||||||
|
|
|
|
|
|
|
(mm/dd/yy) |
(circle one) |
|||||
|
|
|
|
|
|
|
|
|
|
M |
|
F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Address: Street |
|
City |
|
County |
|
State |
|
Zip |
|
|
|||
|
|
|
|
|
|
|
|
TX |
|
|
|
|
|
Signature of person to receive vaccine or person authorized to make the request (parent or guardian): |
|
|
|
|
|||||||||
x |
|
|
|
|
|
|
Date: |
|
|
|
|
||
x |
|
|
|
|
|
|
|
Date: |
|
|
|
|
|
|
Witness |
|
|
|
|
|
|
|
|
|
|
|
|
PRIVACY NOTIFICATION - With few exceptions, you have the right to request and be informed about information that the State of Texas collects about you. You are entitled to receive and review the information upon request. You also have the right to ask the state agency to correct any information that is determined to be incorrect. See http://www.dshs.texas.gov for more information on Privacy Notification. (Reference: Government Code, Section 552.021, 552.023, 559.003, and 559.004)
Privacy Notice: I acknowledge that I have received a copy of my immunization provider’s HIPAA Privacy Notice.
For Clinic / Office Use Only
Clinic / Office Address:
Date Vaccine Administered:
Vaccine Manufacturer:
Vaccine Lot Number:
Site of Injection:
Title of Vaccine Administrator:
Signature of Vaccine Administrator:
Date VIS Given:
Notice: Alterations or changes to this publication is prohibited.
Instructions: File this consent statement in the patient’s chart.
Immunizations |
CDC VIS Revision 10/15/2021 |
|
|
Form Characteristics
| Fact Name | Details |
|---|---|
| Purpose of the EC 106 Form | The EC 106 form serves as an addendum for the Hepatitis B vaccine, detailing essential information about the vaccine, including benefits, risks, and consent for vaccination. |
| Legal Authority | This form is governed by laws pertaining to immunization consent in the state of Texas, specifically referencing Government Code, Section 552.021, 552.023, 559.003, and 559.004. |
| Information Provided | The form requires personal details of the individual receiving the vaccine, as well as the signature of a consenting adult, ensuring that consent is properly documented. |
| Privacy Rights | The form includes a privacy notification, informing individuals of their rights to access and correct personal information collected by the state regarding their immunization records. |
Guidelines on Utilizing Ec 106
Completing the EC 106 form is a straightforward process, but it is essential to provide accurate and clear information. Follow these steps carefully to ensure the form is filled out correctly.
- Begin by reviewing the Vaccine Information Statement (VIS) provided for the Hepatitis B vaccine. Make sure you're familiar with the details.
- In the designated section, clearly write the name of the person receiving the vaccine, including their last name, first name, and middle initial.
- Fill in the birthdate using the format mm/dd/yy.
- Indicate the person's sex by circling either M (Male) or F (Female).
- Provide the complete address of the person receiving the vaccine, including street, city, county, state, and zip code.
- If you are not the person receiving the vaccine, include the signature of the person receiving the vaccine or the parent/guardian authorized to make the request.
- Date the signature by adding the date when permission is granted.
- Provide a witness signature and date it in the appropriate fields.
- Complete the section for clinic or office use, filling out the clinic/office address, date the vaccine was administered, vaccine manufacturer, vaccine lot number, site of injection, and the title and signature of the vaccine administrator.
- Finally, indicate the date VIS was given in the specified area to ensure the information is recorded accurately.
What You Should Know About This Form
What is the EC 106 form?
The EC 106 form is an addendum related to the Hepatitis B vaccine. It serves as a consent statement for individuals receiving the vaccine. This form outlines the rights of the individual, the information provided about the vaccine, and the understanding of its benefits and risks.
Who can sign the EC 106 form?
The form can be signed by the person receiving the vaccine if they are an adult. If the recipient is a minor or unable to provide consent, a parent or legal guardian must sign the form on their behalf.
What information is required on the form?
Key details required include the name, birthdate, address, and sex of the person receiving the vaccine. Also, the individual or guardian’s signature, date, and the name of the vaccine being administered must be recorded.
What does the form state about the risks and benefits of the vaccine?
The EC 106 form emphasizes that the signer understands both the risks associated with the Hepatitis B disease and the benefits and risks of receiving the vaccine. This information is crucial for informed consent.
What should individuals do if they have questions about the vaccine?
The form indicates that individuals should have the opportunity to ask questions regarding the disease, the vaccine itself, and the administration process. It is important to clarify any uncertainties before signing the form.
Is personal information protected when filling out the EC 106 form?
Yes, the EC 106 form includes a privacy notification. Individuals have the right to access their information collected by the State of Texas and can request corrections as needed to ensure accuracy.
What happens after the form is signed?
Once the form is signed, it must be filed in the patient’s chart for record-keeping. The clinic or office staff will also document the details of the vaccine administered, including the date, manufacturer, and lot number.
Can the EC 106 form be altered or changed?
No, alterations or changes to the publication of the EC 106 form are prohibited. This is to ensure that all documentation remains consistent and legally valid.
What is a VIS?
A Vaccine Information Statement (VIS) is a document provided to individuals receiving vaccines. It contains essential information about the vaccine, including benefits, risks, and guidance for the recipient. The EC 106 form confirms that the VIS has been received or offered.
Where can I find more information about privacy and consent?
Additional information about privacy and consent can be found at the Texas Department of State Health Services website. It provides guidelines on privacy notifications and individuals’ rights regarding their health information.
Common mistakes
Filling out the EC 106 form is essential for anyone receiving the Hepatitis B vaccine. However, mistakes can lead to delays or complications in the vaccination process. One common mistake is related to personal information. Many individuals forget to provide complete details, especially the full name and birthdate. Omitting any of this information can result in confusion and may require additional paperwork to correct.
Another frequent error involves the signature. Parents or guardians must sign the form when consenting for a minor. Some people overlook the need for both a parent’s or guardian's signature and their own signature if they are also receiving the vaccine. Neglecting this step can lead to the vaccine not being administered, causing frustration and potential health risks.
Understanding the risks and benefits of the vaccine is crucial. Some applicants only fill out the form without taking the time to read through the Vaccine Information Statement (VIS). This oversight means they may not truly comprehend what they are consenting to, which can have serious implications for their health choices. It's important to ask questions if something is unclear.
Lastly, another mistake is related to the witness signature section. Some individuals fail to include a witness's signature altogether. This oversight is significant as it can invalidate the consent process. Instructing someone to be present during the signing of the form can help ensure that all necessary steps are followed and that the vaccine process goes smoothly.
Documents used along the form
The EC 106 form, which facilitates consent for the Hepatitis B vaccine, is often accompanied by additional documents. These documents help clarify rights, outline procedures, and ensure proper record-keeping.
- Vaccine Information Statement (VIS): This document provides vital information about the vaccine, including potential risks and benefits. It is essential that individuals receive a copy to understand what to expect from vaccination.
- Immunization Record: This record tracks all vaccinations a person has received. It is important for maintaining accurate medical history and ensuring that individuals are up to date on their immunizations.
- Consent for Treatment Form: In some cases, a separate form may be required to document consent for any medical treatment or procedure. This form reinforces the patient's rights and need for informed consent.
- HIPAA Privacy Notice: This document outlines the rights individuals have regarding their personal health information. It explains how health information may be used, shared, and the individual’s rights to privacy.
- Patient Questionnaire: This document may collect relevant health information before vaccination. It assesses medical history and potential risks associated with receiving the vaccine.
- Insurance Authorization Form: If applicable, this form enables healthcare providers to bill insurance for the vaccination. It verifies the patient's insurance and ensures coverage for the procedure.
It's essential to review and understand these forms to maintain clear communication and ensure proper procedures when receiving vaccinations.
Similar forms
- Informed Consent Form: Like the EC 106 form, this document ensures that individuals understand the treatment or procedure being consented to. It requires acknowledgment of risks, benefits, and alternatives before proceeding.
- Vaccine Information Statement (VIS): Similar to the information provided with the EC 106, the VIS explains the risks and benefits associated with vaccines. It is provided to help recipients make informed decisions about vaccination.
- Medical Consent Form: This document, like the EC 106, is used to confirm a patient’s consent for medical procedures. It details the specific treatment and affirms the patient’s understanding of the potential results.
- Release of Information Form: Comparable to the privacy section in the EC 106, this form allows individuals to consent to the sharing of their medical information with designated parties, ensuring compliance with confidentiality laws.
- Immunization Record: This document tracks vaccinations given, similar to the details filled out in the EC 106. It serves as proof that an individual has received specific vaccines and provides essential information for future care.
- Patient Registration Form: Like the EC 106, this form collects personal and demographic information. It helps ensure that medical providers have accurate details about the patient before administering vaccines or treatments.
Dos and Don'ts
When filling out the EC 106 form, it is essential to ensure that the information is accurate and complete. Below is a list of things you should and shouldn't do to help guide you through this process.
- Do ensure all provided information is accurate. Double-check the name, birthdate, and address of the person receiving the vaccine to avoid any mistakes.
- Do read the Vaccine Information Statement (VIS) thoroughly. Understanding the benefits and risks associated with the vaccine is crucial.
- Do sign and date the form correctly. Signatures should be made by the person receiving the vaccine or a legal guardian when applicable.
- Do ask questions. If something is unclear about the vaccine or the information provided, do not hesitate to seek clarification.
- Don't ignore the Privacy Notification. Be aware of your rights concerning personal information and how it is handled.
- Don't forget to mark the vaccination site. It’s important the administrator knows where to administer the vaccine to avoid errors.
- Don't make unauthorized changes to the form. Altering the document could lead to issues with record-keeping or compliance.
Misconceptions
There are several misconceptions regarding the EC 106 form related to the Hepatitis B vaccine. Clarifying these misunderstandings can help individuals navigate the consent process more effectively.
- The form is only for adults. While it requires the signature of a parent or guardian for minors, adults can also consent for themselves using this form.
- This form guarantees vaccine effectiveness. Signing the form does not ensure that the vaccine will be effective for everyone. It is essential to understand the effectiveness is based on medical studies.
- You must have prior knowledge of the vaccine. The form states that individuals should ask questions; therefore, prior knowledge is not required. The opportunity for discussion is provided before signing.
- Signing the form prevents you from asking questions later. Signing the form does not mean that questions cannot be asked in the future. Ongoing communication with healthcare providers is encouraged.
- The privacy notification is optional. The privacy notification included on the form is mandatory. It informs individuals of their rights regarding personal information collected by the state.
Key takeaways
- Consent Requirement: Make sure that you or an authorized person can legally consent to the vaccination for the individual listed on the form.
- Information Statement: Obtain a copy of the Vaccine Information Statement (VIS) for the Hepatitis B vaccine before proceeding with the immunization.
- Understanding Risks: Be aware of the risks associated with Hepatitis B, both the disease and the vaccine.
- Opportunity for Questions: You should take the time to ask questions related to the disease, the benefits, and the administration of the vaccine.
- Personal Identification: Fill out the recipient information accurately, including full name, birth date, sex, and address.
- Documentation: Ensure that a signature from the person receiving the vaccine or their authorized representative is included on the form for validity.
- Privacy Rights: Familiarize yourself with your privacy rights regarding information collected by the State of Texas, including your right to review and correct data.
- HIPAA Compliance: Confirm that you have received your immunization provider’s HIPAA Privacy Notice, which outlines how your information will be used and protected.
- Official Records: The clinic or office must maintain proper documentation of the vaccination, including the date administered and the vaccine lot number.
Browse Other Templates
Disability Determination Office - Respondents should answer all questions accurately to help expedite their claim.
Mdr Philhealth Form - The form requires personal details, including name and birth information.