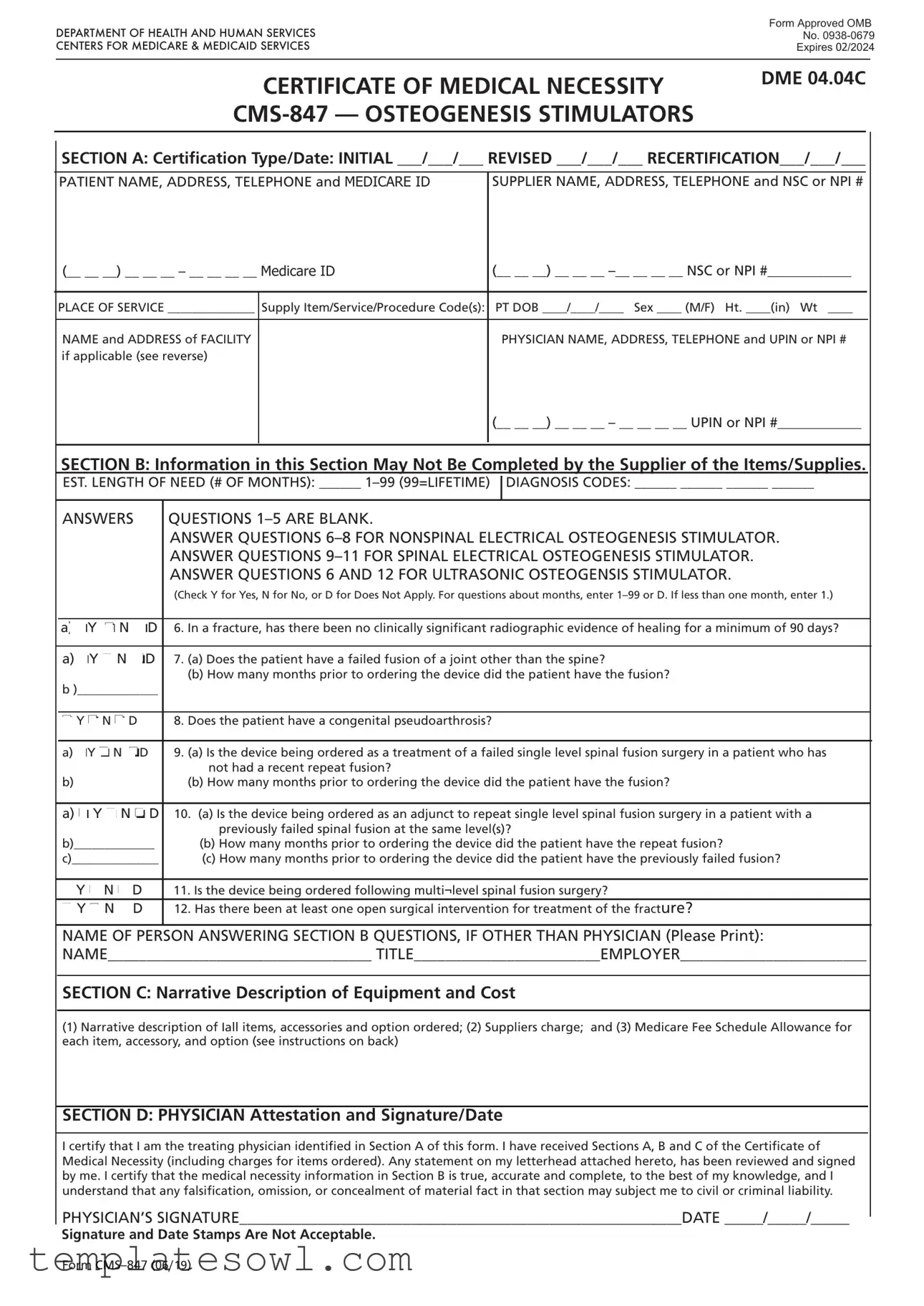
Fill Out Your Cms 847 Form
The CMS 847 form is a critical document in the healthcare field, specifically designed to establish the medical necessity for osteogenesis stimulators, a type of durable medical equipment (DME). Physicians must complete this form to certify that a patient requires these devices, whether for initial treatment, a revision to an existing order, or for recertification of ongoing need. It captures vital information, including patient details such as name, address, Medicare ID, and the supplier's information. The form consists of various sections that outline the type of certification, the duration of need for the equipment, and specific diagnosis codes that justify the request. Furthermore, it includes detailed clinical questions that must be answered to support the medical necessity claim. A physician's signature at the end confirms that all information is accurate and that the request aligns with the patient's medical conditions. By ensuring that every detail is meticulously filled out, healthcare providers can streamline the approval process with Medicare and facilitate access to necessary treatments for their patients.
Cms 847 Example
Form Approved OMB
DEPARTMENT OF HEALTH AND HUMAN SERVICES |
No. |
|
CENTERS FOR MEDICARE & MEDICAID SERVICES |
Expires 02/2024 |
|
|
|
|
CERTIFICATE OF MEDICAL NECESSITY
DME 04.04C
SECTION A: Certification Type/Date: INITIAL ___/___/___ REVISED ___/___/___ RECERTIFICATION___/___/___
PATIENT NAME, ADDRESS, TELEPHONE and MEDICARE ID |
SUPPLIER NAME, ADDRESS, TELEPHONE and NSC or NPI # |
(__ __ __) __ __ __ – __ __ __ __ Medicare ID |
(__ __ __) __ __ __ |
|
|
|
|
PLACE OF SERVICE ______________ |
Supply Item/Service/Procedure Code(s): |
PT DOB ____/____/____ Sex ____ (M/F) Ht. ____(in) Wt ____ |
|
|
|
NAME and ADDRESS of FACILITY |
|
PHYSICIAN NAME, ADDRESS, TELEPHONE and UPIN or NPI # |
if applicable (see reverse) |
|
|
|
|
(__ __ __) __ __ __ – __ __ __ __ UPIN or NPI #____________ |
|
|
|
SECTION B: Information in this Section May Not Be Completed by the Supplier of the Items/Supplies.
EST. LENGTH OF NEED (# OF MONTHS): ______
DIAGNOSIS CODES: ______ ______ ______ ______
ANSWERS |
QUESTIONS |
|
ANSWER QUESTIONS |
|
ANSWER QUESTIONS |
|
ANSWER QUESTIONS 6 AND 12 FOR ULTRASONIC OSTEOGENSIS STIMULATOR. |
|
(Check Y for Yes, N for No, or D for Does Not Apply. For questions about months, enter |
a) oY o N oD 6. In a fracture, has there been no clinically significant radiographic evidence of healing for a minimum of 90 days?
a)oY o N oD 7. (a) Does the patient have a failed fusion of a joint other than the spine?
(b)How many months prior to ordering the device did the patient have the fusion?
b )_____________
o Y o N o D |
8. Does the patient have a congenital pseudoarthrosis? |
a)oY o N oD 9. (a) Is the device being ordered as a treatment of a failed single level spinal fusion surgery in a patient who has not had a recent repeat fusion?
b) |
(b) How many months prior to ordering the device did the patient have the fusion? |
a)o Y o N o D 10. (a) Is the device being ordered as an adjunct to repeat single level spinal fusion surgery in a patient with a previously failed spinal fusion at the same level(s)?
b)_____________ |
(b) How many months prior to ordering the device did the patient have the repeat fusion? |
|
||
c)______________ |
(c) How many months prior to ordering the device did the patient have the previously failed fusion? |
|
||
|
|
|
|
|
o Y |
o |
N o D |
11. Is the device being ordered following multi¬level spinal fusion surgery? |
|
|
|
|
|
|
o Y o N o D |
12. Has there been at least one open surgical intervention for treatment of the fracture? |
|
||
NAME OF PERSON ANSWERING SECTION B QUESTIONS, IF OTHER THAN PHYSICIAN (Please Print):
NAME__________________________________ TITLE________________________EMPLOYER________________________
SECTION C: Narrative Description of Equipment and Cost
(1)Narrative description of Iall items, accessories and option ordered; (2) Suppliers charge; and (3) Medicare Fee Schedule Allowance for each item, accessory, and option (see instructions on back)
SECTION D: PHYSICIAN Attestation and Signature/Date
I certify that I am the treating physician identified in Section A of this form. I have received Sections A, B and C of the Certificate of Medical Necessity (including charges for items ordered). Any statement on my letterhead attached hereto, has been reviewed and signed by me. I certify that the medical necessity information in Section B is true, accurate and complete, to the best of my knowledge, and I understand that any falsification, omission, or concealment of material fact in that section may subject me to civil or criminal liability.
PHYSICIAN’S SIGNATURE_________________________________________________________DATE _____/_____/_____
Signature and Date Stamps Are Not Acceptable.
Form

INSTRUCTIONS FOR COMPLETING THE CERTIFICATE OF MEDICAL NECESSITY
FOR OSTEOGENESIS STIMULATORS
SECTION A: |
(May be completed by the supplier) |
CERTIFICATION |
If this is an initial certification for this patient, indicate this by placing date (MM/DD/YY) needed initially in the space TYPE/ |
DATE: |
marked “INITIAL.” If this is a revised certification (to be completed when the physician changes the order, based on the |
|
patient’s changing clinical needs), indicate the initial date needed in the space marked “INITIAL,” and indicate the |
|
recertification date in the space marked “REVISED.” If this is a recertification, indicate the initial date needed in the |
|
space marked “INITIAL,” and indicate the recertification date in the space marked “RECERTIFICATION.” Whether |
|
submitting a REVISED or a RECERTIFIED CMN, be sure to always furnish the INITIAL date as well as the REVISED or |
|
RECERTIFICATION date. |
PATIENT |
Indicate the patient’s name, permanent legal address, telephone number and his/her Medicare ID as it appears on his/her |
INFORMATION: |
Medicare card and on the claim form. |
SUPPLIER |
Indicate the name of your company (supplier name), address and telephone number along with the Medicare Supplier |
INFORMATION: |
Number assigned to you by the National Supplier Clearinghouse (NSC) or applicable National Provider Identifier (NPI). If |
|
using the NPI Number, indicate this by using the qualifier XX followed by the |
|
e.g. NSC number, use the qualifier 1C followed by the |
PLACE OF SERVICE: |
Indicate the place in which the item is being used, i.e., patient’s home is 12, skilled nursing facility (SNF) is 31, End |
|
Stage Renal Disease (ESRD) facility is 65, etc. Refer to the DMERC supplier manual for a complete list. |
FACILITY NAME: |
If the place of service is a facility, indicate the name and complete address of the facility. |
SUPPLY ITEM/SERVICE |
List all procedure codes for items ordered. Procedure codes that do not require certification should not be listed |
PROCEDURE CODE(S): |
on the CMN. |
PATIENT DOB, HEIGHT, |
Indicate patient’s date of birth (MM/DD/YY) and sex (male or female); height in inches and weight in pounds, if requested. |
WEIGHT AND SEX: |
|
PHYSICIAN NAME, |
Indicate the PHYSICIAN’S name and complete mailing address. |
ADDRESS: |
|
PHYSICIAN |
Accurately indicate the treating physician’s Unique Physician Identification Number (UPIN) or applicable National |
INFORMATION: |
Provider Identifier (NPI). If using the NPI Number, indicate this by using the qualifier XX followed by the |
|
If using UPIN number, use the qualifier 1G followed by the |
PHYSICIAN’S |
Indicate the telephone number where the physician can be contacted (preferably where records would be accessible |
TELEPHONE NO: |
pertaining to this patient) if more information is needed. |
SECTION B: |
(May not be completed by the supplier. While this section may be completed by a |
|
Physician employee, it must be reviewed, and the CMN signed (in Section D) by the treating practitioner.) |
EST. LENGTH OF NEED: |
Indicate the estimated length of need (the length of time the physician expects the patient to require use of the ordered |
|
item) by filling in the appropriate number of months. If the patient will require the item for the duration of his/her life, |
|
then enter “99”. |
DIAGNOSIS CODES: |
In the first space, list the diagnosis code that represents the primary reason for ordering this item. List any additional |
|
diagnosis codes that would further describe the medical need for the item (up to 4 codes). |
QUESTION SECTION: |
This section is used to gather clinical information to help Medicare determine the medical necessity for the item(s) |
|
being ordered. Answer each question which applies to the items ordered, checking “Y” for yes, “N” for no, or “D” for |
|
does not apply. |
NAME OF PERSON |
If a clinical professional other than the treating physician (e.g., home health nurse, physical therapist, dietician) or a |
ANSWERING SECTION B |
physician employee answers the questions of Section B, he/she must print his/her name, give his/her professional title |
QUESTIONS: |
and the name of his/her employer where indicated. If the physician is answering the questions, this space may be |
|
left blank. |
SECTION C: |
(To be completed by the supplier) |
NARRATIVE |
Supplier gives (1) a narrative description of the item(s) ordered, as well as all options, accessories, supplies and drugs; |
DESCRIPTION OF |
(2) the supplier’s charge for each item(s), options, accessories, supplies and drugs; and (3) the Medicare fee schedule |
EQUIPMENT & COST: |
allowance for each item(s), options, accessories, supplies and drugs, if applicable. |
SECTION D: |
(To be completed by the physician) |
PHYSICIAN |
The physician’s signature certifies (1) the CMN which he/she is reviewing includes Sections A, B, C and D; (2) the |
ATTESTATION: |
answers in Section B are correct; and (3) the |
PHYSICIAN SIGNATURE |
After completion and/or review by the physician of Sections A, B and C, the physician’s must sign and date the CMN in |
AND DATE: |
Section D, verifying the Attestation appearing in this Section. The physician’s signature also certifies the items ordered |
|
are medically necessary for this patient. |
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is
DO NOT SUBMIT CLAIMS TO THIS ADDRESS. Please see http://www.medicare.gov/ for information on claim filing.
Form
Form Characteristics
| Fact Name | Description |
|---|---|
| Purpose | The CMS 847 form is used to certify the medical necessity of osteogenesis stimulators for patients, ensuring coverage under Medicare. |
| Sections | The form consists of multiple sections: Section A for certification information, Section B for clinical necessity, Section C for item descriptions and costs, and Section D for physician attestation. |
| Signatures Required | Physicians must sign the form to confirm that the information is accurate and to verify that the items ordered are medically necessary. |
| Expiration | The approval for the CMS 847 form expires in February 2024, after which a new form must be submitted. |
Guidelines on Utilizing Cms 847
Completing the CMS 847 form requires careful attention to detail. This form, also known as the Certificate of Medical Necessity for Osteogenesis Stimulators, must be filled out accurately to ensure proper processing. Next, proceed with the outlined steps to fill out the form completely.
- In Section A, indicate the type of certification by checking the appropriate box for INITIAL, REVISED, or RECERTIFICATION and enter the required dates.
- Provide the patient's name, address, telephone number, and Medicare ID as it appears on the Medicare card.
- Enter the supplier's name, address, telephone number, and their National Supplier Clearinghouse (NSC) or National Provider Identifier (NPI) number.
- Specify the place of service where the item will be used.
- List all supply item/service/procedure codes relevant to the medical need.
- Fill in the patient's date of birth, sex, height, and weight.
- Include the physician's name, address, telephone number, and UPIN or NPI number.
- In Section B, enter the estimated length of need in months (1-99).
- Provide diagnosis codes that justify the medical need for the item (up to 4 codes).
- Answer the applicable questions in Section B, checking Y for Yes, N for No, or D for Does Not Apply.
- Include the name of the person answering Section B questions if it is not the physician.
- In Section C, the supplier should write a narrative description of all items, the supplier's charge, and the Medicare Fee Schedule allowance.
- In Section D, the physician must print their name, sign, and date, certifying the accuracy of the information provided.
What You Should Know About This Form
What is the CMS-847 form used for?
The CMS-847 form, known as the Certificate of Medical Necessity, is specifically used for the prescription of osteogenesis stimulators. It certifies that the requested medical equipment is necessary for the patient's treatment and provides relevant information for Medicare to determine eligibility for coverage.
Who needs to fill out the CMS-847 form?
The CMS-847 form must be completed by the treating physician and may also require input from the supplier of the equipment. Certain sections may not be completed by the supplier, particularly Section B, which addresses medical necessity and clinical details.
What sections are included in the CMS-847 form?
The CMS-847 form consists of four sections: Section A captures patient and supplier information, Section B gathers clinical information related to medical necessity, Section C details the equipment description and costs, and Section D is for the physician's attestation and signature.
How do I determine the estimated length of need on the CMS-847 form?
In Section B, you should indicate the expected duration a patient will require the osteogenesis stimulator. This is documented by filling in the number of months needed, which can range from 1 to 99, where "99" signifies a lifetime need.
What types of information need to be provided about the patient?
Essential information about the patient includes their name, legal address, telephone number, date of birth, sex, height, weight, and Medicare ID. This ensures accurate identification and facilitates the processing of the claim.
How should diagnosis codes be entered on the CMS-847 form?
In Section B, you will input the diagnosis codes relevant to the patient’s condition. List the primary diagnosis followed by any additional codes that reflect the necessary medical need. A maximum of four codes should be provided.
What happens if the physician's information is incorrect on the form?
If the physician's information is inaccurate or incomplete, it may lead to processing delays or denials of coverage by Medicare. It is crucial for the physician to review all entries carefully before signing the form.
What signature is required on the CMS-847 form?
The physician must sign and date Section D of the CMS-847 form. This signature attests that the information provided is truthful and certifies the medical necessity of the ordered items. Stamps or electronic signatures are not accepted.
How long is the CMS-847 form valid?
The CMS-847 form is only valid until the expiration date specified on the form, which, in this case, is February 2024. It is important to ensure that any submissions are made before this date to avoid issues with coverage or reimbursement.
Common mistakes
Filling out the CMS 847 form can be a straightforward process if you avoid common mistakes. One mistake is leaving the certification type and date section blank or incomplete. Each section—initial, revised, and recertification—requires accurate dates, so ensure that this is filled out correctly to avoid delays.
Another frequent error involves the patient's information. Omitting or incorrectly entering details such as the name, address, telephone number, and Medicare ID can lead to processing issues. It’s vital to double-check that all this information matches the patient's records exactly.
Individuals often neglect to provide diagnosis codes accurately. This section requires up to four codes that justify the medical necessity of the device. Skipping this or including incorrect codes can result in unnecessary denials of claims.
Many people mistakenly leave critical questions in Section B unanswered. Each question helps to establish the medical necessity for the device. Failing to respond to applicable questions may trigger a denial, so be sure to answer all that relate to the patient's situation.
Another oversight can occur in the narrative description section. A vague or incomplete narrative about the equipment ordered can lead to confusion and potential claim rejections, affecting timely access to necessary medical devices.
Errors often stem from not identifying the length of need in Section B correctly. The number of months must be entered; a common mistake is writing zero or omitting this completely, which can signal a lack of medical necessity.
Physician signatures are crucial. Some mistakenly assume they can use signature stamps. Signatures and dates must be personally written by the physician to validate the document fully. The absence of a proper signature can result in the rejection of the form.
Finally, individuals sometimes misunderstand the attestation process. It is important that the physician comprehensively review all sections of the form before signing. Failing to do this may create liability risks and improperly processed claims, affecting patient care.
Documents used along the form
The CMS 847 form, known as the Certificate of Medical Necessity for Osteogenesis Stimulators, is often accompanied by a variety of other forms and documents. These documents help streamline the approval process for medical devices and ensure that all necessary information is captured accurately. Below is a list of commonly used forms that support the CMS 847.
- CMS 1500 Form: This is a standard claim form used by healthcare providers to bill Medicare and other payers for medical services and supplies. It captures patient and provider information as well as diagnosis and procedure codes.
- CMS 484 Form: This form is used for Home Health Certification and Plan of Care. It outlines the medical necessity of home health services and includes the physician's signature, which is essential for Medicare coverage.
- ABN (Advance Beneficiary Notice of Noncoverage): This notice informs patients that Medicare may not cover a specific service or treatment. It allows patients to make informed decisions about their care and potential costs associated with it.
- Prescription for Medical Equipment: This document, provided by the healthcare provider, indicates that a specific medical device or supply is necessary for the patient’s treatment. It is often required for insurance claims.
- Physician's Order: This written directive from a physician includes instructions for the treatment or supply needed by the patient. It serves as a critical component in demonstrating medical necessity.
- Medical Records: These records provide comprehensive details about the patient’s diagnosis and treatment history, establishing the medical need for the services or equipment being requested.
- Prior Authorization Form: This form is sometimes required by insurers before certain treatments, devices, or medications are provided. It ensures that the service is medically necessary under the insurance policy guidelines.
- CMS 855 Form: This application for enrollment in the Medicare program provides information about the supplier or provider, helping to establish eligibility and legitimacy before claims are processed.
- Enrollment Forms for Patients: These typically capture patient demographic and insurance information to assist healthcare providers in obtaining necessary authorizations and billing correctly.
- Detailed Product Descriptions: Suppliers may provide detailed descriptions of the medical equipment, including prices and specifications, to justify medical necessity and reimbursement amounts under Medicare guidelines.
Using these forms together with the CMS 847 can facilitate a smoother process when applying for coverage of medical equipment and ensure that all necessary information is available for review. Understanding and preparing these documents in advance can ease the burden on both patients and healthcare providers.
Similar forms
- CMS-486 - This form is used to document the medical necessity for durable medical equipment (DME). Similar to the CMS-847, it requires patient and supplier information as well as the physician’s signature.
- CMS-835 - This form is also related to DME need and clinical documentation. Like the CMS-847, it focuses on justifying the necessity based on the patient’s health condition.
- CMS-1500 - This claim form is used for billing Medicare. It shares similarities with the CMS-847 in that both require patient and provider details, along with a description of the medical service or equipment.
- CMS-25 - Used for outpatient services treatment, this form also requires a physician's input. Like the CMS-847, it specifies patient diagnosis and treatment details.
- ECF-1500 - Primarily for mental health claims, this form requires similar documentation as the CMS-847, including patient and provider information alongside clinical necessity.
- CMS-3172 - This form handles prior authorization for medical services like the CMS-847. It requires diagnosis codes and clinical information from the physician.
- CMS-849 - This form assists with chronic disease management. It requires patient history and a physician’s statement, akin to the process outlined in the CMS-847.
- CMS-1023 - Used for enrollment as a Medicare provider, it similarly requires comprehensive provider information for verification purposes like the supplier's data in the CMS-847.
- CMS-855I - This form is for Medicare enrollment and includes extensive details about the healthcare provider, paralleling the detailed identification process in the CMS-847.
- CMS-625 - This form supports requests for durable medical equipment. It includes clinical justification requirements similar to those found in the CMS-847.
Dos and Don'ts
- Do ensure that all patient information is filled out accurately, including Medicare ID and contact details.
- Don’t leave any questions in the certification section unanswered, as this may delay the process.
- Do indicate whether it is an initial, revised, or recertification request clearly in Section A.
- Don’t forget to have the physician review and sign the form, as their verification is crucial.
Misconceptions
Misconceptions about the CMS 847 form can lead to confusion and errors in the medical billing process. Here are eight common misconceptions along with clarifying explanations:
- The CMS 847 form is only for Medicare patients. This form is primarily used for Medicare, but it can also apply to Medicaid or private insurance, depending on the requirements of the payer.
- Only doctors can fill out the CMS 847 form. While a physician's signature is required for final approval, non-physician clinicians may assist in completing certain sections, as long as the physician reviews and signs off on it.
- The CMS 847 form doesn't require specific diagnoses. On the contrary, valid diagnosis codes must be included to justify the medical necessity of the items or services ordered.
- The length of need must always be 99 months. This is incorrect. The length of need can vary based on the patient's condition and should reflect the anticipated duration of necessity, with 99 indicating lifetime use.
- Completing the form is the supplier's responsibility. Not exactly; the supplier can gather information, but it is the physician who must certify and sign the form. Their oversight is crucial for compliance.
- The CMS 847 form is unchangeable once submitted. This is a myth. If there are changes in a patient's condition, a revised or recertified form can be submitted to reflect new information.
- The narrative description is optional. This is false. A detailed narrative description of the equipment, its cost, and any accessories is required to ensure clear communication of medical necessity.
- Section B can be filled out by anyone. Section B specifically must be answered by a qualified professional, and it should be reviewed and signed by the treating physician to ensure accuracy.
Understanding these misconceptions can streamline the process and enhance the accuracy of submissions, leading to better patient care and compliance with Medicare regulations.
Key takeaways
- The CMS 847 form is specifically designed to certify the medical necessity of osteogenesis stimulators.
- This form must be carefully filled out to ensure that Medicare can approve the required medical equipment.
- Section A allows for the entry of patient details, including their Medicare ID and the supplier's information.
- It is crucial to denote the type of certification—whether initial, revised, or recertification—accurately.
- Section B should be completed only by a physician or authorized medical staff, not by the supplier.
- Diagnoses must be clearly documented using the specified codes to substantiate the medical need for the device.
- Specific questions in Section B ascertain the patient's medical condition and treatment history, and must be answered diligently.
- In Section C, a detailed narrative description of the ordered items, including prices, is required.
- The physician's signature in Section D confirms the accuracy of the provided information and validates the necessity of the equipment.
- It is vital to ensure that the CMN is completed and signed correctly to avoid any delays in authorization or service provision.
Browse Other Templates
Free Farm Lease Agreement Forms to Print - By detailing expectations, this lease helps to foster a strong landlord-tenant relationship.
Guardian Shield Application,Sentinel Security Form,Defender Entry Document,Vigilant Guard Application,Protector Security Registration,Safeguard Employment Form,Watchman Application Sheet,SecureShield Candidate Form,Overwatch Security Application,Fort - Marksman Security emphasizes the importance of transparency in the hiring process.
Property Tax Write Off - Deductible auto expenses include gas, wear and tear, and repairs for business use.