Fill Out Your Fsis 7234 1 Form
The FSIS 7234-1 form is a vital component in the process of obtaining USDA approval for labels, markings, and devices associated with poultry, egg, and meat products. This form requires detailed information from applicants, including the area of the principal display panel and the total labeling space for the entire package. Applicants must specify the type of product they are submitting, whether it falls under different categories like slaughter, raw, or thermally processed items. Furthermore, the form addresses critical aspects such as whether the label has been previously approved, any special claims or guarantees set forth, and the inclusion of foreign languages on the label. It’s important to note that certain portions of the form, particularly items 11, 15, and 16, are exempt from public disclosure under the Freedom of Information Act, ensuring that sensitive information remains protected. Applicants must also include their firm's name and contact information, along with a signature and date, affirming the authenticity of the submission. The overall process is designed to ensure that all labeling conforms to USDA standards, thereby maximizing safety and transparency for consumers. Understanding how to properly complete this form can help streamline the approval process and reduce delays in getting your products to market.
Fsis 7234 1 Example
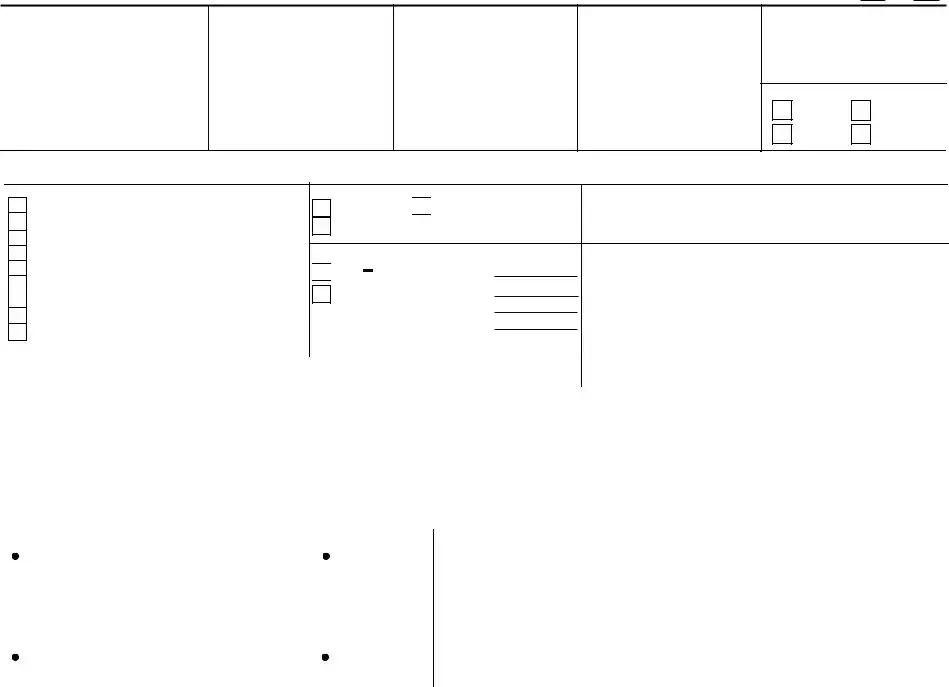
According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is
U.S. DEPARTMENT OF AGRICULTURE
FOOD SAFETY AND INSPECTION SERVICE
APPLICATION FOR
APPROVAL OF LABELS,
MARKING OR DEVICE
FSIS has determined that information provided in items 11, 15, and 16 is exempt from mandatory disclosure under the Freedom of Information Act 5 U.S.C. 552(b)(4).
APPLICANT: See Page 5 for instructions.
1.AGENT NAME, ADDRESS, TELEPHONE NO. (If using an Agent, complete this block, otherwise leave blank.)
2. FOR USDA USE ONLY
5a. NAME OF PRODUCT
5b. HACCP PROCESS CATEGORY (Select one)
03J: Slaughter - all species
03B: Raw Product - ground
03C: Raw Product - not ground
03D: Thermally processed - commercially sterile
03E: Not heat treated - shelf stable
6a. TYPE OF APPROVAL REQUESTED
SKETCH 
 TEMPORARY
TEMPORARY
EXTENSION OF TEMPORARY
6b. WAS THE LABEL PREVIOUSLY APPROVED? 
 YES ►Date of approval:
YES ►Date of approval:
03F: Heat treated - shelf stable  03G: Fully cooked - not shelf stable
03G: Fully cooked - not shelf stable
03H: Heat treated but not fully cooked - not shelf stable
03I: Product with secondary inhibitors - not shelf stable
NO
Prior approval number: Number of labels on hand: Number of days requested:
|
|
8. Does this label include a |
|
|
Yes |
|
No |
|
|
9. (FOR |
|||||||
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
10. Are there any special claims, guarantees, or foreign language on the label? |
|
|
YES |
|
NO (If yes, check all that apply) |
|
|
|
|||||||
|
|
|
|
|
|
|
|
||||||||||
|
|
|
Allergen Statements |
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
Other Claims: Specify |
|
|
|
|||
|
|
|
Animal Production/Breed/Raising |
|
|
Grading Terms |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
Certified/Verified |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
Guarantees |
|
|
|
|
|
|
|
|
||||
|
|
|
Environmental/Green |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
Natural/Organic |
|
|
|
|
|
|
|
|
||||
|
|
|
Export Only Labels w/deviations from Domestic Requirements |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign Language |
|
|
Nutrition/Health |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
Geographic/Undefined Style |
|
|
Religious Exemption |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
11. NAME AND ADDRESS OF FIRM (Below and between dots) |
|
|
|
|
|
|
12. SIGNATURE OF APPLICANT OR AGENT |
13. DATE |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
14. (FOR USDA USE ONLY) CONDITIONS APPLYING TO USE OF LABELS OR DEVICE |
|||||
FSIS FORM |
REPLACES FSIS FORM |
FSIS FORM
15. PRODUCT FORMULA
PAGE of
PCT |
|
WEIGHT |
(No Fractions)
|
|
See Continuation Sheet |
TOTAL (Percent must total 100%) |
|
|
|
|
FSIS FORM |
PAGE |
|
of |
|
|
|
|
16. PROCESSING PROCEDURES (Approval of the sketch does not convey approval of the processing procedures)

 See Continuation Sheet
See Continuation Sheet

FSIS FORM |
PAGE |
|
of |
|
|
|
|
||||
|
|
|
|
|
|
CONTINUATION SHEET FOR APPLICATION FOR APPROVAL OF LABELS,
MARKING OR DEVICE (FSIS
PRODUCT NAME:
This sheet is being used for additional information required in Block(s):
INSTRUCTIONS FOR PREPARATION OF FSIS FORM
Note: The following instructions should be typed unless otherwise noted.
A. |
PREPARATION OF APPLICATION |
7b. |
Total available labeling space in square inches for entire package. |
|
Application must be typed or it will be returned without evaluation. |
8. |
|
|
Submit two copies for each label application. |
||
|
|
|
includes a |
B. |
TYPE OF APPROVAL REQUESTED |
9. |
Leave Blank. For |
|
Sketch: Self explanatory. (See 9 CFR 317.4 & 381.132) |
||
|
|
|
|
|
Temporary and Extension of Temporary. Actual label or color litho take off to be used. |
10. |
Special claims, guarantees, or foreign language. Indicate if there are |
|
|
|
are any special claims, guarantees, or foreign language on the label. |
C. |
FOREIGN LANGUAGE |
|
Check all that apply. If Other Claims is selected, indicate specific claim(s) |
|
Labels printed in foreign languages must be accompanied by English language translation. |
|
|
|
|
in space provided. |
|
|
|
|
|
D. |
ASSEMBLY OF APPLICATION |
11. |
Name and Address of Firm. Insert Firm's name and mailing |
|
Application Form, Product Formula, Processing Procedures, Continuation Sheet if applicable, Label, |
|
address. Use 2 letter symbol for State. Show postal zip code. |
|
and any Supporting Documentation Staple with one or as few staples as possible. (Do not use paper |
12 & 13. |
Signature and Date of Applicant or Agent. To be signed and |
|
clips). |
||
|
|
|
dated by the applicant or agent representing the official |
E. |
MAIL COMPLETED APPLICATION TO: |
|
establishment or plant. |
|
|
||
|
USDA, FSIS, OPPD, LPDD |
14. |
|
|
Labeling Distribution Unit |
Leave blank for USDA use only. Conditions Applying to Use of Label or |
|
|
|
||
|
Stop Code 3786, Patriots Plaza III, |
|
Device. (Any condition, modification or remarks applied to the application |
|
1400 Independence Avenue, SW |
|
when approved are conditions governing use of the approved devices.) |
|
Washington, DC |
|
|
The following instructions relate to numbered items on form. |
15. |
Product Formula. List the ingredients by percent or weight in order of |
|
1. |
If using an Agent, provide the company name, address, and telephone number, |
|
their predominance. If product consists of several components, e.g., |
|
a frozen dinner, list each component separately and indicate the |
||
|
otherwise leave blank. |
|
percentage or amount of each component in the product. If additional |
2 & 3. |
Leave blank, for USDA use only. |
|
space is needed, check the box for "Continuation Sheet," and use the |
|
Continuation Sheet. Be sure to include the product name and number |
||
|
|
|
of the block item. Express all ingredients in the same units, i.e., do not |
4. |
Establishment No./Foreign Country (if applicable) - Self Explanatory. |
|
list some in pounds and others in ounces. |
4a. |
Type of Product. Select one product type: Egg, Meat, Poultry, or Other (i.e. Exotic Species, |
|
|
|
Check whether weight or percent is used. It is preferred that |
||
|
|
||
5a. |
Name of Product. Use common or descriptive product name, i.e., "Frankfurter , |
|
percentages be used, and the total must equal 100 percent. If weights |
|
are used, show in pounds, kilograms or grams. (No gallons, pints, |
||
|
Cereal Added" or "Meat Patties in Gravy. (Do not use trade brand names or |
|
|
|
|
cups, teaspoons, etc.) The total must equal the weights of the |
|
|
coined names, such as "Joe's Corn Dogs" or "Joe's Sloppy Joes.") If coined |
|
|
|
|
individual units. (Example: Crust + Cheese + Sauce + Meat = Total |
|
|
names such as "Corn Dogs" are used, also show true product name, such as |
|
|
|
|
new weight of unit.) |
|
|
"Batter Wrapped Wiener." |
|
|
|
|
|
|
5b. |
Provide HACCP process category for the product. See 9 CFR 417.2(b) (1), |
|
DO NOT use fractions. Express as decimals carried to two places, Example: |
|
Example, Heat Treated - shelf stable, Not heat |
|
|
6a & b. |
Type of Approval Requested. If temporary approval or extension, insert |
|
|
|
number of days requested and number of labels on hand. If previous |
|
|
|
approval, attach copy of application and label. Include specific reason(s) |
16. |
Processing Procedures. Poultry Products provide complete |
|
why requesting a temporary or extension and include information required |
||
|
in 9 CFR 317.4(f) (1) or 381.132(f) (1) on the continuation sheet. |
|
processing procedures as required in 9 CFR 381.134. Meat |
|
Be sure to include product name and block item. |
|
Products, provide complete processing procedures as required. |
|
|
|
Note: Approval of the sketch does not convey approval of the |
7a. |
Area of Principal Display Panel (PDP). The PDP is the entire side |
|
processing procedures. If additional space is needed, check the box for |
|
"Continuation Sheet," and use the Continuation Sheet. |
||
of the package to which the label is affixed. See 9 CFR 317.2 (d) |
Be sure to include the product name and number of the block item. |
|
and 381.116 (b). |
||
|
||
|
|
|
FSIS FORM |
|
Form Characteristics
| Fact Name | Fact Description |
|---|---|
| Form Purpose | The FSIS Form 7234-1 is used for applying for approval of labels, markings, or devices for poultry, meat, and egg products. |
| Labeling Space | It requires the applicant to specify the area of the principal display panel as well as the total available labeling space for the entire package. |
| OMB Control Number | The valid OMB control number for this information collection is 0583-0092, as mandated by the Paperwork Reduction Act of 1995. |
| Completion Time | Completing the FSIS Form 7234-1 typically takes approximately 75 minutes, including instruction review and data gathering. |
| USDA Use Only | Certain sections of the form, indicated as "FOR USDA USE ONLY," are exclusively for the USDA to fill out. |
| Information Confidentiality | FSIS has exempted information provided in items 11, 15, and 16 from mandatory disclosure under the Freedom of Information Act. |
| Applicant Information | Applicants must provide their name, address, and, if applicable, the name of their agent, as well as the agent's contact information. |
| Product Type | Applicants are required to classify their product as poultry, meat, egg, or other, including various subclasses of each. |
| Approval Types | The form allows submission for temporary approval or extension of previously granted approvals for labeling. |
| Processing Procedures | It stipulates that approval of product sketches does not imply approval of processing procedures, which also must be submitted for review. |
Guidelines on Utilizing Fsis 7234 1
Completing the FSIS Form 7234-1 is an important step in ensuring that your product meets labeling requirements. After filling out the form, you will submit it to the USDA for approval. The following steps will guide you through the process of filling out the form correctly.
- In Block 1, provide the agent's name, address, and telephone number if applicable. If not using an agent, leave this block blank.
- Leave Block 2 and Block 3 blank; these are for USDA use only.
- In Block 4, enter the establishment number or foreign country information if applicable.
- In Block 4a, select the type of product: Poultry, Other, Egg, or Meat.
- In Block 5a, write the common name of the product, such as "Frankfurter with Cereal Added."
- In Block 5b, choose the applicable HACCP process category from the provided options.
- In Block 6a, indicate the type of approval requested (e.g., Temporary, Extension of Temporary).
- In Block 6b, if the label was previously approved, enter the date of approval or a prior approval number. Otherwise, leave it blank.
- In Block 7a, measure and enter the area of the principal display panel in square inches.
- In Block 7b, provide the total available labeling space for the entire package in square inches.
- In Block 8, check 'Yes' or 'No' regarding the inclusion of the USDA-AMS Child Nutrition Program Logo.
- Block 9 is for USDA use only; leave it blank.
- In Block 10, indicate 'Yes' or 'No' for special claims, guarantees, or foreign languages on the label. If applicable, check all that apply.
- In Block 11, provide the firm’s name and address, using the proper format.
- In Block 12, have the applicant or agent sign the form.
- In Block 13, enter the date the form is signed.
- Leave Block 14 blank; this section is for USDA use only.
- In Block 15, list the product formula, ensuring percentages total 100% without fractions.
- In Block 16, describe the processing procedures clearly and comprehensively, using the continuation sheet if necessary.
Make sure to review the form thoroughly before submitting. It is important to ensure all information is accurate and complete to avoid delays. Once finalized, submit the completed form along with any necessary attachments to the designated USDA address.
What You Should Know About This Form
What is the FSIS Form 7234-1 used for?
The FSIS Form 7234-1 is an application used to obtain approval for labels, markings, or devices for meat, poultry, and egg products. This form is essential for producers and manufacturers to ensure compliance with USDA labeling requirements. By submitting this form, applicants provide vital information about their products, how they are processed, and any special claims made on the label. Approval ensures that these products meet safety and labeling standards before they hit the market.
How long does it take to complete the FSIS 7234-1 form?
Completing the FSIS 7234-1 form is estimated to take about 75 minutes on average. This time includes gathering necessary information, following instructions, and filling out the various sections of the form. It's important to set aside enough time to review all details carefully, as inaccuracies could delay approval processes.
What information is specifically required on the FSIS Form 7234-1?
The FSIS Form 7234-1 requires several key pieces of information such as the applicant's details, establishment number, product type, and a description of the product. Additionally, applicants must specify the HACCP process category, declare any special claims or guarantees, and include the area of the principal display panel. Each section asks for clear and precise information to ensure correct processing and approval of the application.
Can I submit the FSIS Form 7234-1 online?
Currently, the FSIS Form 7234-1 can be submitted through various channels, but it does not support direct online submission. Applicants typically need to print the completed form and send it physically to the USDA. Once you prepare your application, ensure that all the components—including supporting documents—are appropriately stapled and sent to the designated USDA office for processing.
What should I do if my label is denied approval?
If your label application is denied, don't be discouraged. The FSIS typically provides reasons for the disapproval, which can help you understand the specific issues. It's important to review the feedback closely, make the necessary adjustments, and then resubmit your application. Additionally, leveraging resources such as USDA guidelines and possibly consulting with a labeling expert can improve your chances of approval in the next round.
Common mistakes
Filling out the FSIS Form 7234-1 can feel overwhelming, but many people make common mistakes that can lead to delays in approval. One prevalent error is failing to provide complete contact information. Whether you’re the applicant or using an agent, it’s vital to include the full name, address, and telephone number. Leaving any of these details out can cause a hold-up in processing your form.
Another frequent mistake involves the inaccurate measurement of labeling space. Section 7a requires you to measure the area of the principal display panel in square inches, but some applicants either miscalculate or misinterpret the instructions. Ensuring accurate measurements helps USDA evaluate your label’s compliance more effectively and speeds up the review process.
Many people also overlook the importance of specifying the type of product in Section 5a. It’s more than just a technicality. Using descriptors like “Frankfurter” or “Meat Patties in Gravy” is essential. Vague names or trade brand names can lead to complications. Providing a clear product name helps USDA’s review team classify and assess your application properly.
Another critical error is not selecting the HACCP process category correctly in Section 5b. This selection significantly affects your approval because the category governs how your product is processed. Make sure to reference the provided guidelines for correct options, as this step is pivotal in ensuring food safety standards.
Also, neglecting to include any required attachments can hinder your application. If you’ve indicated prior approval in Section 6b, ensure to attach the previous approval number or a copy of the prior label. This additional documentation can demonstrate the continuity of compliance, which is valuable during the review process.
Finally, it’s easy to confuse the signature and date sections. These must be completed accurately by either the applicant or an authorized agent. Omitting a signature or date can lead to automatic rejection of your application, so it's important to double-check these critical fields. Taking the time to avoid these errors can mean a smoother experience with your FSIS Form 7234-1 submission.
Documents used along the form
The FSIS 7234-1 form plays a crucial role in the approval process for meat, poultry, and egg Product labels. It ensures that products comply with regulations set forth by the USDA's Food Safety and Inspection Service (FSIS). However, it is often accompanied by other important documents that help provide a comprehensive view of the product and its labeling. Understanding these supplementary documents is essential for compliance and effective communication with regulatory bodies.
- FSIS Form 7202: This form is used for the application of export certification. It verifies that the packaged product meets the importing country's health and safety standards.
- FSIS Form 7022: A labeling application form for products needing approval outside of the typical criteria. It is particularly relevant for specific consumer concerns, such as dietary restrictions.
- Product Formulation Sheet: It details the composition of the product, including all ingredients and their respective percentages. This allows FSIS to assess the product's compliance with labeling standards.
- Continuity Form: This document is used to provide ongoing details related to any changes or modifications to approved labels and ensure accuracy in labeling over time.
- HACCP Plan: The Hazard Analysis and Critical Control Points (HACCP) Plan outlines the safety protocols in place during production. It demonstrates a commitment to food safety and quality assurance.
- Supplemental Labeling Information: This form provides extra details about the product's claims, such as nutritional information or allergen statements. It supports compliance with the labeling regulations.
- Labeling Claim Documentation: Necessary for products making specific claims, this document backs up claims related to organic, non-GMO, or gluten-free statuses, ensuring transparency and trustworthiness.
- Foreign Language Labeling Requests: If a product label is in a foreign language, this document must accompany the application. It ensures compliance with regulations regarding bilingual labeling.
- Inspection Reports: These reports provide details from facility inspections by FSIS. They verify that the establishment meets standards for hygiene, safety, and overall quality control before products are released.
- Withdrawal Request Forms: If changes are made to a label post-approval, this form is used to withdraw the previously approved label to avoid any confusion or compliance issues in the market.
When submitting the FSIS 7234-1 form, it's essential to understand the accompanying documents that enhance the application process. Together, these forms create a robust framework for ensuring the integrity and compliance of food labeling, promoting consumer safety and informed choices. Having a clear grasp of this process can significantly aid food businesses in navigating the regulatory landscape effectively.
Similar forms
The FSIS Form 7234-1 is essential for the approval of labels, markings, or devices in the food industry. Several other documents demonstrate similar purposes or processes. Below is a list of these documents and how they relate to the FSIS Form 7234-1:
- FSIS Form 7234-2: This form is used for applications involving temporary approval or extension of labels. Like the 7234-1, it requires specifics about the product, including labeling space and the nature of the product.
- FDA Form 2579: This form is utilized for food labeling requirements at the federal level. It shares similarities with FSIS Form 7234-1 in that both require details about product ingredients, claims, and labeling space to ensure compliance with regulations.
- USDA Grain Inspection, Packers and Stockyards Administration (GIPSA) Form 36: This document requests labeling approval for grain products. Similar to FSIS Form 7234-1, it collects information on labeling practices and ingredient transparency.
- FSIS Form 2000-1: Designed to report HACCP plan records, this form mirrors the 7234-1 in emphasizing food safety. Both documents are critical for ensuring compliance with federal food safety regulations.
- FSIS Form 9100: This application form is for new meat and poultry products. It closely relates to FSIS Form 7234-1 as it also requires detailed information about product safety, labeling, and manufacturing processes.
- Nutrition Labeling Exemption Application (FDA): While it specifically addresses nutrition labeling, this application shares similarities with FSIS Form 7234-1 in its need for thorough documentation regarding product composition and marketing claims.
Understanding these forms and their purposes can help companies navigate the regulatory landscape more effectively.
Dos and Don'ts
When filling out the FSIS 7234-1 form, there are important guidelines to keep in mind. Here are ten things you should and shouldn't do:
- Do ensure the application is typed to avoid rejection.
- Don't use trade brand names; instead, use common product names.
- Do provide accurate information in all required fields.
- Don't leave any sections blank unless specified.
- Do indicate if your product includes a USDA-AMS Child Nutrition Program Logo.
- Don't forget to sign and date the application before submission.
- Do submit two copies of the application for each label request.
- Don't mix measurement units; keep them consistent throughout the form.
- Do clearly outline any special claims or guarantees on the label.
- Don't forget to include your establishment number if applicable.
Following these suggestions will help ensure a smoother application process. If any confusion arises, seeking clarification can be beneficial.
Misconceptions
- Misconception 1: The FSIS 7234-1 form is only for poultry products.
- Misconception 2: Filling out the form takes a lot of time.
- Misconception 3: You cannot submit the form if you don’t have a previous approval number.
- Misconception 4: USDA needs a hard copy only.
- Misconception 5: The application must be hand-written.
- Misconception 6: Special claims on labels are not allowed.
- Misconception 7: You must complete the entire form if it's not applicable to your product.
This form actually applies to a variety of products, including meat, eggs, and poultry. It's essential to understand that multiple categories exist within the form, allowing for a broad range of submissions.
While it may seem daunting, the average time to complete this form is estimated at 75 minutes. This includes reviewing instructions and gathering information, making it manageable for most applicants.
If your label has never been approved before, you can leave that section blank. The form is designed to accommodate new applications without prior records.
Although traditionally submitted in hard copy, the form has been approved for web distribution. Applicants can, and should, check current guidelines for submission methods.
The form must be typed. Handwritten submissions may be returned without evaluation. Typing ensures that the information is clear and legible.
Special claims, guarantees, or the use of foreign languages can be included on the label. The key is to indicate these clearly on the form and select the applicable options.
It's okay to leave sections blank if they do not apply to your product. This flexibility helps streamline the application process for everyone involved.
Key takeaways
Here are key takeaways regarding the filling out and using the FSIS 7234-1 form:
- The form is primarily used for the approval of labels, markings, or devices related to various meat and poultry products.
- Applicants must include their establishment number or foreign country information if applicable.
- Process categories must be selected from a provided list, such as "Raw Product - ground" or "Fully cooked - not shelf stable."
- The area of the principal display panel (PDP) and total available labeling space must be accurately measured and recorded.
- Submitting two copies of the application is required for each label being applied for.
- Approval conditions, once granted, govern the use of approved labels, which should be followed closely.
- Special claims or features, like allergen statements, must be clearly indicated on the form if they appear on the label.
Understanding each section of the FSIS 7234-1 form is crucial for compliance and approval of product labeling. Be thorough in your documentation to ensure a smooth review process.
Browse Other Templates
Checkbook Register Template Pdf - Encourage healthier financial habits by tracking your spending diligently.
Donor Profile - Current donor level indicating engagement with the organization.
