Fill Out Your Generic Request Form
The Generic Request form is an essential tool used in the Department of Defense TRICARE pharmacy program for obtaining prior authorization for brand name medications when a generic alternative is available but deemed unsuitable. This form must be completed and signed by a healthcare prescriber, ensuring that the necessary patient and physician information is clearly provided, including the patient's name, date of birth, sponsor ID, and contact details. The provider plays a vital role in initiating this process by selecting the appropriate medication and outlining any clinical justifications for prescribing a brand name drug instead of an A-rated generic. Operating under specific guidelines, the form requires a thorough consideration of the patient's experiences with generic medications and any adverse reactions they may have encountered. Patients must use the provided contact methods, such as faxing the form or sending it via mail to Express Scripts, which processes these requests. This ensures an organized way for healthcare professionals and patients to navigate the prior authorization process effectively.
Generic Request Example
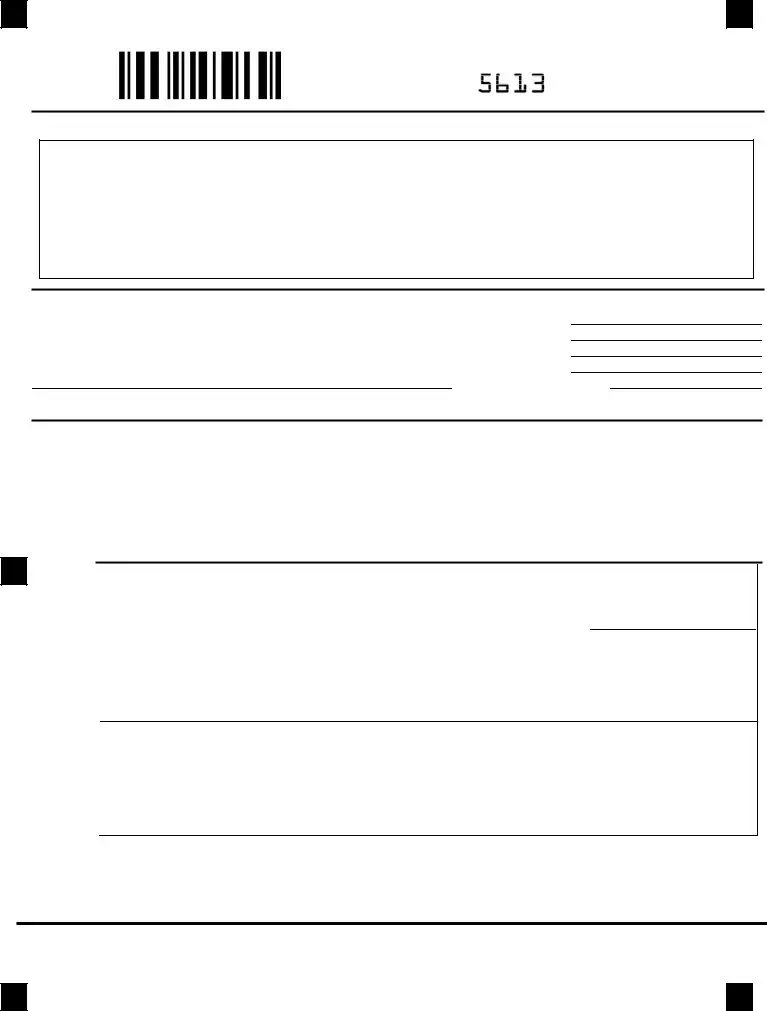
Brand over Generic Prior Authorization Request Form
To be completed and signed by the prescriber. To be used only for prescriptions which are to be filled through the Department of Defense (DoD) TRICARE pharmacy program (TPHARM). Express Scripts is the TPHARM contractor for DoD.
The provider may call:
The patient may attach the completed form
to the prescription and mail it to: Express Scripts, P.O. Box 52150, Phoenix, AZ
or email the form only to:
Step
1
Please complete patient and physician information (Please Print)
Patient Name: |
|
Physi cian Name: |
Address: |
|
Address: |
|
||
Sponsor ID # |
|
Phone #: |
|
||
Date of Birth: |
|
Secure Fax #: |
Please indicate which medication is being prescribed: ________________________________________________
Step
2
Please consider the following:
32 CFR 199.21 (j)(2) Use of generic drugs under the pharmacy benefits program. The pharmacy benefits program generally requires mandatory substitution of generic drugs listed with an “A” rating in the current Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book) published by the FDA and generic equivalents of grandfather or Drug Efficacy Study Implementation (DESI) category drugs for brand name drugs. In cases in which there is a clinical justification for a brand name drug in lieu of a generic equivalent, under the standards and procedures of paragraph (h)(3) of this section, the generic substitution policy is waived.
The generic products are
1. Has the patient tried the generic product? |
Yes |
|
Proceed to Question 2 |
2. Did the patient experience a significant adverse |
Yes |
reaction to the generic? |
Proceed to Question 3 |
No
Proceed to Question 4
No
Proceed to Question 3
3.Please provide an explanation of the patient’s experience with the generic, then proceed to Step 3:
4.Please provide
Step |
I certify that the above is correct and accurate to the best of my knowledge. Please sign and date: |
|
3 |
________________________________________ |
___________________ |
|
Prescriber Signature |
Date |
[ 5 May 2018 ]
Form Characteristics
| Fact Name | Detail |
|---|---|
| Purpose | The Generic Request form is used to request prior authorization for brand name medications when generics are clinically inappropriate. |
| Governing Law | The process is governed by 32 CFR 199.21(j)(2), which addresses the use of generic drugs under the DoD pharmacy benefits program. |
| Prescriber Requirement | The form must be completed and signed by a prescriber to ensure validity and accountability. |
| Submission Methods | Prescribers can submit the form via fax or patients can mail or email it, providing flexibility in submission. |
| Contractor Information | Express Scripts is the designated contractor managing the TRICARE pharmacy program (TPHARM) for the DoD. |
| Patient Information | Essential patient details such as name, date of birth, and sponsor ID must be included for proper processing. |
| Adverse Reactions | The form requires documentation if the patient has had significant adverse reactions to previously tried generic products. |
| Clinical Justification | If a generic cannot be used, the form must outline patient-specific clinical reasons to justify the request for a brand name. |
| Signature Requirement | A prescriber’s signature and date are mandatory to validate the request, confirming the information provided is accurate to the best of their knowledge. |
Guidelines on Utilizing Generic Request
Filling out the Generic Request form is a straightforward process that ensures proper communication between prescribers, patients, and the pharmacy program. Follow the steps carefully to ensure that all necessary information is accurately provided, facilitating a smooth approval process for the prescribed medication.
- Complete Patient and Physician Information: Begin by entering the required details in clear, legible handwriting.
- Patient Name
- Physician Name
- Address
- Sponsor ID #
- Phone #
- Date of Birth
- Secure Fax #
- Indicate the Medication: Clearly state the name of the medication being prescribed next to the section that asks for this information.
- Consider Key Questions: Reflect on the following points regarding the patient’s experience with the generic product:
- Has the patient tried the generic product? (Yes or No)
- If Yes, consider whether the patient experienced a significant adverse reaction to the generic. (Yes or No)
- Should you provide an explanation based on the patient's experience or specific clinical justification? Do this as prompted.
- Certify Information: Confirm the accuracy of the provided information and sign the form. Include the date next to your signature.
Once the form is completed, you can either fax it or send it via mail or email following the contact details provided on the form. This ensures that your request is promptly attended to by the appropriate pharmacy contractor, thereby assisting in the provision of necessary medications for your patients.
What You Should Know About This Form
What is the purpose of the Generic Request form?
The Generic Request form is designed for prescribers to request a brand name medication when there is a clinical justification for not using a generic equivalent. This form is specifically used for prescriptions that will be filled through the Department of Defense (DoD) TRICARE pharmacy program. By submitting this form, patients can ensure that the necessary documentation is in place for the approval of brand medications when warranted.
Who should fill out the Generic Request form?
The Generic Request form must be completed and signed by the prescriber. This includes filling out important details about the patient, the physician, and the medication being prescribed. Both the physician and the patient need to provide accurate information to streamline the approval process.
How can I submit the Generic Request form?
There are several options for submitting the Generic Request form. The prescriber can either call Express Scripts at 1-866-684-4488 or fax the completed form to 1-866-684-4477. Alternatively, the patient can attach the completed form to their prescription and mail it to Express Scripts at P.O. Box 52150, Phoenix, AZ 85072-9954. Email submissions are also accepted at TpharmPA@express-scripts.com, but it is crucial that only the form is sent via email.
What information is required on the form?
When completing the Generic Request form, essential information must be provided. This includes the patient's name, date of birth, and Sponsor ID number, as well as the prescriber’s name and contact information. The specific medication being prescribed must also be clearly indicated. Ensuring this information is accurate will assist in a smoother processing of the request.
What if the patient has previously tried the generic medication?
If the patient has already tried the generic medication, the form will guide the prescriber through additional questions. If the patient experienced significant adverse reactions, the prescriber must provide an explanation. If the patient did not have issues with the generic, there needs to be a specific patient-related clinical justification for why the A-rated generic cannot be used. This step is critical for the approval process and must be thoroughly documented.
Common mistakes
Completing the Generic Request form requires meticulous attention to detail. Start with a common mistake: failing to provide complete patient and physician information. Each field marked with asterisks needs to be filled out accurately. Missing or incorrect entries can delay the processing of the request, potentially impacting patient care.
Another frequent error is poor legibility. It is crucial to print clearly. If the information cannot be read, it can lead to misunderstandings or, worse, a rejection of the request. Always use a pen with black or blue ink, as light colors may not photocopy well and could create further complications.
Some individuals neglect to check the patient's previous use of the generic product. It is vital to establish whether the patient has tried the alternative before proceeding. Not answering this question can lead to unnecessary back-and-forth communication, slowing down the approval process.
Equally important is the failure to document significant adverse reactions accurately. If a patient has had an adverse reaction to the generic, it must be noted explicitly. Skipping this step can result in the prescriber’s justification lacking the necessary support.
Inadequate explanations for why the A-rated generic cannot be used also pose an issue. Generic Request forms often face scrutiny due to insufficient clinical justification. It is expected that prescribers detail why a brand-name drug is more suitable, based on the specific circumstances of the patient’s health.
Submitting the form without a signature is a mistake one should not make. The prescriber’s signature and date serve as a validation of the provided information. An unsigned form is incomplete and will inevitably lead to delays.
Moreover, not verifying the contact details for the office is a common lapse. Ensure that the phone number and fax number are correct and functional. Clear lines of communication are essential when the pharmacy or insurance has questions about the request.
Patients often overlook the mailing process. Remember to check that the completed form is attached to the prescription before sending it out. Failure to do so may mean the request gets separated from the medication, leading to miscommunication.
Not providing an appropriate fax number or e-mail can also result in complications. If you're sending electronically, confirm that the email address is correct. A small typo in an email can send important documentation into the void, resulting in delays.
Lastly, keeping outdated information or using old forms can lead to confusion and rejection. Always ensure you are using the most current version of the form, which incorporates changes to policies or guidelines that could influence the approval process.
Documents used along the form
When submitting a Generic Request form, several other documents may accompany it to ensure the process is thorough and all necessary information is provided. Each of these forms plays a unique role in supporting the request for prior authorization or clarifying patient needs.
- Patient Medical History Form: This document outlines the patient’s medical background and any previous medications taken. It helps the prescriber justify the need for a brand name over a generic drug based on the patient's history.
- Prescriber Letter of Medical Necessity: A letter penned by the prescriber explaining why a specific brand name medication is necessary for the patient's treatment. This letter typically includes details about adverse reactions to generics or unique patient circumstances.
- Pharmacy Benefit Management Form: Often required by insurance companies, this form documents the patient's insurance coverage and any previous claims about medications. It ensures that all billing procedures align with the patient's pharmacy benefits.
- Adverse Reaction Report: If the patient experienced side effects from a generic medication, this report details those reactions. It serves to reinforce the need for a brand name alternative, providing evidence to support the request.
- Prior Authorization Tracking Form: This tracking form helps manage the authorization process. It includes dates, contact information, and any communications between the provider and the insurance company, which ensures transparency and accountability throughout the process.
Submitting the Generic Request form alongside these documents can facilitate a smoother approval process. By providing detailed information, you strengthen the case for the necessary medication, which can significantly impact patient care.
Similar forms
- Prior Authorization Request Form - Similar in purpose, this document is submitted to obtain approval before a medication can be filled. Both forms require completion by the prescriber and focus on justifying the necessity of a brand name over a generic.
- Medical Necessity Form - This form supports the need for specific treatment and may require similar patient and physician information. It allows for documentation of the medical justification for choosing one medication over another, aligning with the goals of the Generic Request form.
- Prescription Prior Authorization Form - This document serves to formally request prior authorization for prescription drugs. Like the Generic Request form, it necessitates detailed information about the patient, prescriber, and specific medication involved, and explains the reason for the choice of medication.
- Drug Exception Request - This document is comparable as it seeks an exception for a patient to receive a brand medication when generics are typically prescribed. Both forms capture clinical information to justify this request and require a prescriber’s signature.
- Specialty Pharmacy Request Form - It requests approval for specialized drugs, akin to exceptions in the Generic Request form. Each form requires thorough details, including the patient’s history with other medications.
- Out-of-Network Authorization Request Form - This document seeks permission to obtain coverage for medications from out-of-network providers. Its requirement for explicit rationale mimics the need for justification in the Generic Request form.
- Formulary Exception Request Form - Patients may use this form to request coverage for medications not included on the formulary. Similar to the Generic Request form, it requires a prescriber’s input on why a non-formulary item is necessary.
- Claim Reconsideration Form - Used to appeal an unsuccessful claim for medication coverage, this document shares the need for detailed explanations and justifications that align with the patient’s treatment plan, much like the Generic Request form.
Dos and Don'ts
When filling out the Generic Request form, there are important guidelines to follow to ensure that the process goes smoothly. Both patients and prescribers play a key role in this process. Below are some helpful do's and don'ts.
- Do ensure that all patient and physician information is completed accurately and clearly. Use print letters to avoid any confusion.
- Do state specifically which medication is being prescribed. This helps avoid any potential misunderstandings or delays.
- Do provide detailed explanations in the sections regarding adverse reactions or clinical justifications. Clear explanations are crucial for approval.
- Do check the form for completeness before submitting it. Missing information can hinder the process.
- Don't leave any sections blank. Incomplete forms may be returned or delayed.
- Don't use ambiguous language when detailing the patient’s experience or needs. Be as specific as possible.
- Don't neglect to sign and date the form. An unsigned form cannot be processed.
- Don't forget to verify that you are mailing the form to the correct address or faxing it to the right number. Double-check this information!
Misconceptions
- Misconception 1: The Generic Request form is only for patients who cannot tolerate generic medications.
- Misconception 2: Submitting the form guarantees approval for the brand-name medication.
- Misconception 3: The process is the same for all medications.
- Misconception 4: Patients do not need to be involved in the submission process.
- Misconception 5: All generic medications require this form for brand-name alternatives.
- Misconception 6: The prescriber signature is optional.
- Misconception 7: Only the prescriber can submit the form.
- Misconception 8: The form must be mailed; faxing is not an option.
This is not entirely accurate. The form is meant for any patient who requires a brand-name drug instead of a generic, regardless of whether they have experienced intolerance.
Approval is not guaranteed. The prescriber must provide adequate clinical justification for the brand-name medication.
Different medications may have varying requirements for approval, depending on their classifications and equivalences determined by regulatory bodies.
Patients may need to attach the completed form to their prescription and send it to the designated address. Their involvement is important to ensure timely processing.
This form is specifically for cases where clinical justification is necessary. Not all situations require its use.
This is incorrect. The prescriber must sign and date the form as an affirmation of the provided information's accuracy and completeness.
While the prescriber completes the form, patients may also submit it, either by mailing or emailing, as specified in the instructions.
Faxing the completed form is indeed an option to expedite processing, offering flexibility for prescribers.
Key takeaways
Utilizing the Generic Request Form involves several important steps and considerations. Here are ten key takeaways to keep in mind when filling it out and using it effectively:
- The form must be completed and signed by the prescriber.
- It is specifically for prescriptions to be filled through the Department of Defense (DoD) TRICARE pharmacy program.
- Express Scripts operates as the TRICARE pharmacy contractor for the DoD.
- Providers can call 1-866-684-4488 or fax the completed form to 1-866-684-4477 for inquiries or assistance.
- Patients should attach the completed form to their prescription, which can be mailed to Express Scripts at P.O. Box 52150, Phoenix, AZ 85072-9954.
- Alternatively, patients have the option to email the form to TpharmPA@express-scripts.com.
- When completing the form, all patient and physician information should be printed clearly.
- Ensure that you provide the patient's name, sponsor ID, phone number, and date of birth accurately.
- Identify the medication being prescribed in the designated section of the form.
- Clinical justification is required if the prescriber determines that a brand-name drug is necessary instead of the A-rated generic alternative.
Following these key points will help streamline the process and ensure that all necessary information is included.
Browse Other Templates
Provider Update Form - This process helps Johns Hopkins keep a reliable provider directory.
Do You Have to Have a Front License Plate in Missouri - The Missouri Lien Form is essential for both individual and business transactions involving vehicles.
