Fill Out Your Prime Therapeutics Pa Form
The Prime Therapeutics PA form serves as a crucial resource for members and healthcare providers regarding the pharmacy benefits managed by Blue Cross and Blue Shield of Florida, Inc. (BCBSF). Since its implementation on January 1, 2012, the form details various programs aimed at optimizing medication management, including Prior Authorization, Responsible Step, and Responsible Quantity programs. Members may encounter changes in coverage for specific oral oncology medications, which now require prior authorization to ensure that treatments align with established clinical guidelines and product labeling. The document outlines a list of affected medications and emphasizes the importance of patient-specific documentation for approval. Additionally, it introduces limitations on certain prescription drugs under the Responsible Quantity program, thereby ensuring adherence to FDA dosing guidelines. This helps in maintaining the quality of care while aiming to curb unnecessary expenditures. As the landscape of medication coverage evolves, ongoing communication between members and their healthcare providers is essential for navigating these changes effectively.
Prime Therapeutics Pa Example
Page 1 of 6
Effective January 1, 2012, Blue Cross and Blue Shield of Florida, Inc. (BCBSF) and Health Options, Inc. will expand our Responsible Rx pharmacy program for BlueCare, BlueChoice and BlueOptions. Responsible Rx refers to an umbrella of programs including Prior Authorization, Responsible Step and Responsible Quantity programs. Members with endorsements that support these programs may be affected.
Prior Authorization
Effective January 1, 2012, the medications listed below will require prior authorization for coverage under the member’s pharmacy benefit.
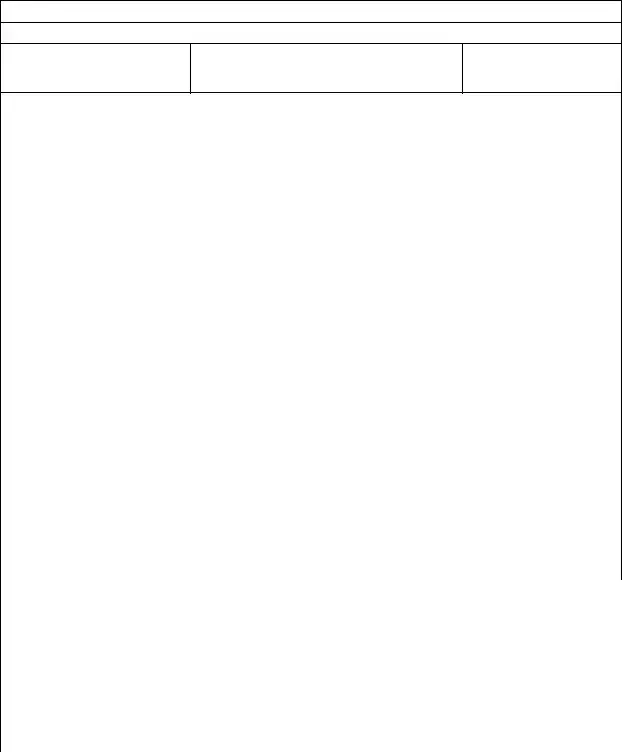
Oral Oncology
Afinitor® (everolimus) |
Nexavar® (sorafenib) |
Temodar® (emozolomide) |
Xeloda® (capecitabine) |
Caprelsa® (vandetanib) |
Oforta® (fludarabine) |
Targretin® (bexarotene) |
Zelboraf® (vemurafenib) |
Gleevec® (imatinib) |
Revlimid® (lenalidomide) |
Thalomid (thalidomide) |
Zolinza® (vorinostat) |
Hexalen® (altretamine) |
Sprycel® (dasatinib) |
Tretinoin® (oral) |
Zytiga® (abiraterone) |
Hycamtin® (topotecan) |
Sutent® (sunitinib) |
Tykerb® (lapatinib) |
Sylatron® (peginterferon) |
Matulane® (procarbazine) |
Tarceva® (erlotinib) |
Votrient® (pazopanib) |
|
Lysodren® (mitotane) |
Tasigna® (nilotinib) |
Xalkori® (crizotinib) |
|
The intent of the Oral Oncology Agents Prior Authorization (PA) program is to ensure appropriate selection of patients for treatment according to product labeling and/or clinical studies and clinical guidelines. Patients currently prescribed therapy with one of these agents will be able to continue their established therapy. New prescriptions after January 1, 2012 will require review.
Pharmaceutical compendia [National Comprehensive Cancer Network (NCCN) Drugs & Biologics Compendium, American Hospital Formulary Service (AHFS) Drug Information, DrugDex, and Clinical Pharmacology] may be consulted to evaluate for medically accepted
BCBSFL will also be classifying these oral oncology medications as specialty pharmacy medications, effective January 1, 2012. BCBSF’s preferred specialty pharmacy provider is CVS Caremark Specialty Pharmacy. CVS Caremark’s toll free number is
of pocket cost share when using an
New Drugs to Existing Programs
Orencia subcutaneous® (abatacept)
Orencia subcutaneous will be added to our Immunomodulators PA program.
Prior authorization request forms are available on the provider website at www.bcbsfl.com under For Providers, then Pharmacy, and the Prior Authorization Program Information and Authorization forms link.
Page 2 of 6
Responsible Quantity Expansion
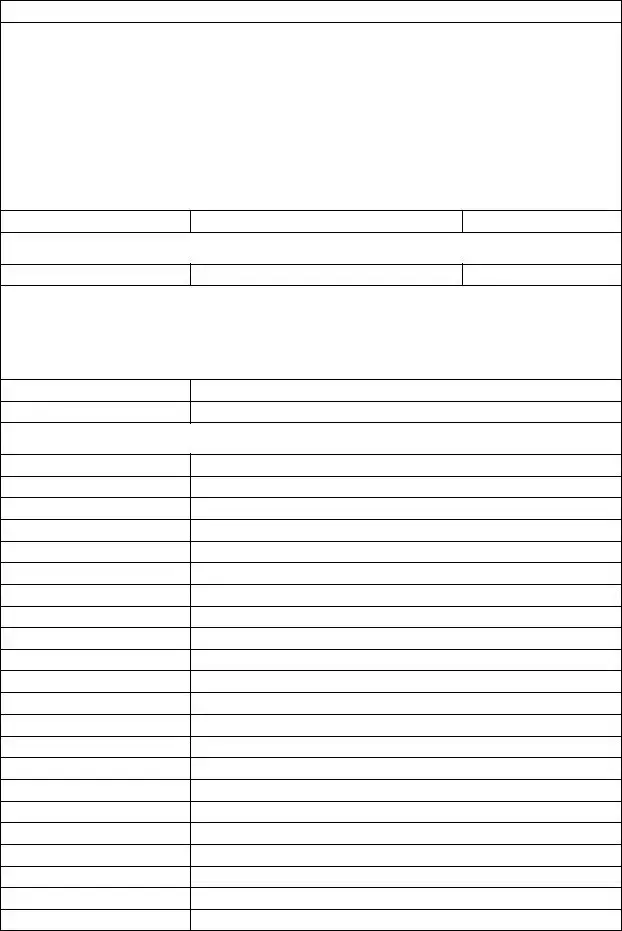
This program ensures coverage of certain prescription drugs that reflect dosing guidelines of drug manufacturers and the U.S. Food and Drug Administration (FDA). The table below lists all additional medications and limits added to the Responsible Quantity Program effective January 1, 2012. This only applies to members in plans that are part of the Responsible Quantity Program. You can find a complete list of prescription drugs modified in the program at www.bcbsfl.com; select For Providers, Pharmacy, and then click the Responsible Quantity Program Information link.
Additions to the Responsible Quantity Program Effective 1/1/12
Responsible Quantity Program limits also apply to generic drugs where applicable
Brand/ Generic Name
Antidepressants
Strength
Dispensing Limit
Per Month
(unless noted)
Aplenzin |
|
30 tabs |
|
|
|
bupropion |
75 mg |
60 tabs |
|
|
|
bupropion |
100 mg |
120 tabs |
|
|
|
bupropion SR |
100 mg, 150 mg, 200 mg |
60 tabs |
|
|
|
bupropion ER |
150 mg, 300 mg |
30 tabs |
|
|
|
Celexa (citalopram) |
tablets |
30 tabs |
|
|
|
Celexa (citalopram) |
oral solution |
600 mL |
|
|
|
fluvoxamine |
25 mg, 50 mg |
30 caps |
|
|
|
fluvoxamine |
100 mg |
90 caps |
|
|
|
Lexapro |
tablets |
30 tabs |
|
|
|
Lexapro |
oral solution |
600mL |
|
|
|
Luvox CR |
|
60 caps |
|
|
|
Maprotiline |
|
90 tabs |
|
|
|
mirtazapine |
7.5 mg, 15 mg, 30 mg, 45 mg |
30 tabs |
|
|
|
Oleptro |
150 mg |
45 tabs |
|
|
|
Oleptro |
300 mg |
30 tabs |
|
|
|
Paxil (paroxetine) |
10 mg, 20 mg, 40mg |
30 tabs |
|
|
|
Paxil (paroxetine) |
30 mg |
60 tabs |
|
|
|
Paxil (paroxetine) |
oral suspension |
900 mL |
|
|
|
Paxil CR (paroxetine ER) |
12.5 mg |
30 tabs |
|
|
|
Paxil CR (paroxetine ER) |
25 mg, 37.5 mg |
60 tabs |
|
|
|
Pexeva (paroxetine) |
10 mg, 20 mg, 40 mg |
30 tabs |
|
|
|
Pexeva (paroxetine) |
30 mg |
60 tabs |
|
|
|
Prozac (fluoxetine) |
10 mg |
30 caps/tabs |
|
|
|
Prozac (fluoxetine) |
20 mg |
120 caps/tabs |
|
|
|
Prozac (fluoxetine) |
40mg |
60 caps |
|
|
|
fluoxetine |
60 mg |
30 tabs |
|
|
|
Prozac (fluoxetine) |
oral solution |
600 mL |
|
|
|
Prozac weekly (fluoxetine) |
|
4 caps / 28 days |
|
|
|
Page 3 of 6
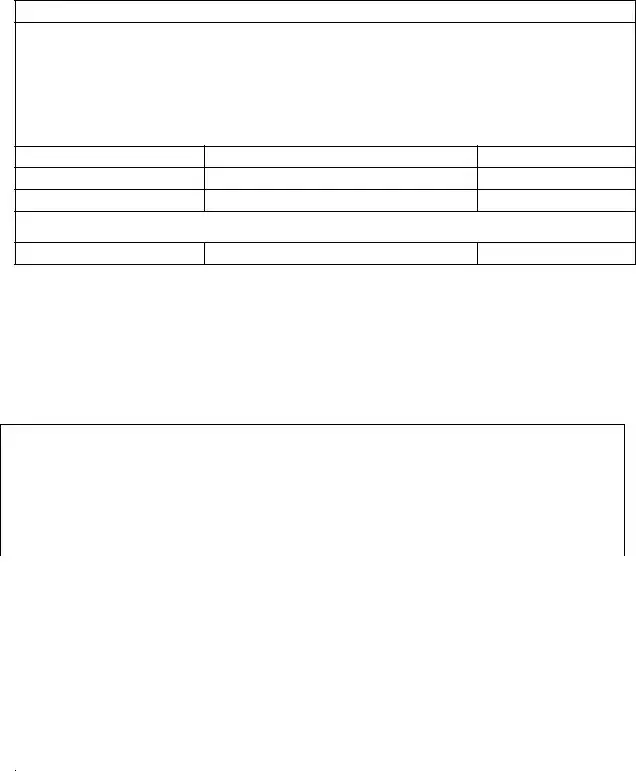
Additions to the Responsible Quantity Program Effective 1/1/12
Responsible Quantity Program limits also apply to generic drugs where applicable
|
|
Dispensing Limit |
Brand/ Generic Name |
Strength |
Per Month |
|
|
(unless noted) |
Viibryd |
10 mg, 20 mg, 40 mg |
30 tabs |
|
|
|
Zoloft (sertraline) |
25 mg, 50 mg |
30 tabs |
|
|
|
Zoloft (sertraline) |
100 mg |
60 tabs |
|
|
|
Zoloft (sertraline) |
oral concentrate |
300 mL |
|
|
|
|
|
Dificid
40 tabs / 180 days
Orencia subcutaneous
4 syringes / 28 days
Diabetes
Juvisync |
100 mg/10 mg, 100 mg/20 mg, 100 mg/40 |
30 tabs |
|
(sitagliptin/simvastatin) |
mg |
||
|
Hematology
Xarelto
Xarelto
10 mg |
35 tabs / 90 days |
15 mg and 20 mg |
30 tabs |
|
|
Oral Oncology
Afinitor
Caprelsa
Caprelsa
Gleevec
Gleevec
Nexavar
Revlimid
Revlimid
Sprycel
Sprycel
Sutent
Sutent
Tarceva
Tarceva
Tasigna
Thalomid
Thalomid
Tykerb
Votrient
Xalkori
Zelboraf Zolinza
|
30 tablets |
100 mg |
60 tabs |
300 mg |
30 tabs |
100 mg |
90 tabs |
400 mg |
60 tabs |
|
120 tabs |
5 mg, 10 mg |
30 caps |
15 mg, 25 mg |
21 caps/28 days |
20 mg |
60 tabs |
50 mg, 70 mg, 80 mg, 100 mg, 140 mg |
30 tabs |
12.5 mg |
90 caps |
25 mg, 50 mg |
60 caps |
25 mg |
60 tabs |
100 mg, 150 mg |
30 tabs |
|
120 caps |
50 mg, 100 mg |
30 caps |
150 mg, 200 mg |
60 caps |
|
180 tabs |
|
120 tabs |
|
60 caps |
|
240 tabs |
|
120 caps |
|
|
Page 4 of 6
Additions to the Responsible Quantity Program Effective 1/1/12
Responsible Quantity Program limits also apply to generic drugs where applicable
|
|
Dispensing Limit |
Brand/ Generic Name |
Strength |
Per Month |
|
|
(unless noted) |
Zytiga |
|
120 tabs |
|
|
|
Pain
Conzip
Nucynta ER
Suboxone/Subutex
30tabs
60tabs
15 tabs / 90 days
Vaccines
Influenza
1 / 90 days
For members requiring a larger monthly quantity than the coverage maximum, based on medical necessity, you may submit a prior authorization request by filling out the Quantity Limit Prior Authorization form at www.bcbsfl.com; select For Providers and then Pharmacy.
Responsible Steps Expansion
Drugs included in Responsible Steps Program and qualifying prerequisites beginning
1/1/12
|
New Programs |
Prerequisite(s) |
Hyalgan, Orthovisc, Supartz* |
Euflexxa, Synvisc, Synvisc One |
|
|
|
|
Juvisync |
metformin, sulfonylureas, metformin/TZD |
|
|
|
combination, metformin/sulfonylurea |
|
|
combination, sulfonylurea/TZD combination |
*this program is part of our Medical Step Therapy program and only applies medical claims for members in new HMO products that support medical step therapy
Authorization request forms are available on the provider website at www.bcbsfl.com under For Providers, then Pharmacy, then the Responsible Steps Program Information and Authorization Forms link.
Pharmacy Coverage Exclusions
Effective JANUARY 1, 2012, BCBSF commercial pharmacy plans will no longer cover the brand name drugs listed in the table below. However, BCBSF will cover many of their generic alternatives. This exclusion only applies to members in plans that allow pharmacy coverage exclusions.
Drugs not covered |
|
Covered alternatives |
|
|
|
Benzoyl Peroxide Wash 7% & Cream 5.5% Kit |
|
benzoyl peroxide (Rx only) |
|
|
|
Veltin gel, Ziana gel |
|
clindamycin phosphate topical gel 1%, tretinoin |
|
|
topical gel 0.025% |
|
|
|

Page 5 of 6
Responsible Rx Expansion
QUESTIONS AND ANSWERS
Responsible Quantity
QUESTION: Why are there limits on the quantity that I can get on my prescription?
ANSWER: BlueCross and BlueShield of Florida has established quantity limits or coverage maximums for certain medications based on manufacturers and FDA approved dosing guidelines.
QUESTION: Will there be limits on all of my medicines?
ANSWER: Only medications included in the Responsible Quantity Program have quantity limits.
QUESTION: My doctor said I had to have this many pills. What can I do?
ANSWER: Responsible Quantity does not prevent you from receiving the medicine your doctor has prescribed for you. The program places a coverage maximum on select medicines filled in a

Page 6 of 6
Responsible Steps
QUESTION: I received a letter that said I would no longer have coverage for my medication unless I have tried a generic first. I do not like to use generics because I have read that they are not as effective as brand drugs.
ANSWER:
Generic drugs are
QUESTION: I have asked my doctor about generics and he/she put me on the brand drug that you are no longer covering. What can I do?
ANSWER: Check with your doctor if a generic drug is right for you. If not, authorization forms for drugs in our Responsible Steps program are available on our website. Your doctor will fill out the form and fax it in for review. Forms are available at www.bcbsfl.com under providers, then pharmacy, then Responsible Steps Program Information and Authorization Forms.
QUESTION: I have taken the prerequisite meds in the past, but it has been longer than the time period required in the responsible steps program. Can I still qualify for coverage?
ANSWER: In order for your claim for a responsible steps drug to be covered, you will need to have a claim for a prerequisite drug within the last 90 days.
QUESTION: It says that current users will not be affected by this step. Does this mean that no matter what, I can still get my prescription filled?
ANSWER: No. In order for your claim for your drug to be covered, you will need to have a claim in our system for a prerequisite drug within the last 90 days. In other words, you have to keep refilling your prescription regularly.
Form Characteristics
| Fact Name | Details |
|---|---|
| Prior Authorization Requirement | Certain oral oncology medications require prior authorization for coverage starting January 1, 2012. |
| Specialty Pharmacy Provider | BCBSF's preferred specialty pharmacy provider is CVS Caremark Specialty Pharmacy, reachable at 1-866-278-5108. |
| Responsible Quantity Program | This program ensures coverage of prescription drugs based on FDA dosing guidelines and restricts dispensing limits for specific medications. |
| New Drugs Addition | Orencia subcutaneous® (abatacept) was added to the Immunomodulators Prior Authorization program. |
| Pharmacy Coverage Exclusions | Effective January 1, 2012, certain brand-name drugs will no longer be covered under BCBSF commercial pharmacy plans. |
| Quantity Limits Reasoning | Limits are established to align with the manufacturers' and FDA approved dosing guidelines, ensuring proper medication use. |
| Patient Continuity in Therapy | Patients already prescribed specific therapies can continue their treatment uninterrupted, even if new prescriptions require a review. |
| Medical Necessity Appeals | Members requiring more than the allowed quantity may submit a prior authorization request based on medical necessity. |
Guidelines on Utilizing Prime Therapeutics Pa
Filling out the Prime Therapeutics Prior Authorization (PA) form is an essential step to ensure that your request for medication coverage is processed efficiently. Here’s how to effectively complete the form:
- Obtain the Form: Access the Prime Therapeutics PA form from the provider website at www.bcbsfl.com. Navigate to the "For Providers" section and select "Pharmacy," then find the "Prior Authorization Program Information and Authorization forms" link.
- Patient Information: Fill in the patient's information, including their full name, date of birth, and patient ID number.
- Provider Information: Enter the healthcare provider's details, such as the name, phone number, and NPI (National Provider Identifier) number.
- Medication Details: Specify the medication for which you are requesting prior authorization. Include the drug name, dosage, and frequency of administration.
- Clinical Information: Provide relevant clinical information that supports the need for the medication. This may include diagnoses, previous treatments, and any supporting documentation or notes from the patient's medical history.
- Sign and Date: The healthcare provider must sign and date the form to verify the accuracy of the information provided.
- Submission: Submit the completed PA form via the designated channels, whether by fax, mail, or online submission as outlined on the provider website.
After submitting the form, the request will be reviewed. You will receive notification regarding the approval or denial of the authorization. This decision is crucial for the patient's access to necessary medications and treatment plans.
What You Should Know About This Form
What is the Prime Therapeutics PA form?
The Prime Therapeutics PA form is used to request prior authorization for certain medications under the pharmacy benefit program. This form is necessary when a member is prescribed specific medications that require approval before they can be covered by insurance. By filling out this form, healthcare providers can initiate the review process for the prescribed medication, ensuring that it meets the necessary criteria for coverage.
Why are there limits on the quantity of medication I can receive?
Limits on medication quantities are established by BlueCross and BlueShield of Florida based on manufacturer guidelines and FDA-approved dosing instructions. These limits ensure that medications are prescribed and used in a safe and effective manner. While some medications are affected, many prescriptions still remain available without such restrictions.
Does the quantity limit apply to all medications?
No, quantity limits are specific to medications within the Responsible Quantity Program. Only those drugs flagged within this program have defined limits. Routine prescriptions not included will not face these restrictions.
What should I do if my doctor prescribes more medication than the limit?
If your doctor prescribes a quantity that exceeds the coverage maximum, you can still receive your medication. You will be charged your usual copayment or coinsurance for the amount covered by the plan. For any excess medication needed, your doctor can request a prior authorization to review and potentially approve the higher quantity for you.
How can I access the Prior Authorization forms?
To obtain the Prior Authorization forms, visit the BlueCross and BlueShield of Florida provider website. This section is clearly marked under the "For Providers" tab, where you can navigate to "Pharmacy" and then to "Prior Authorization Program Information" to find the necessary forms.
What happens if I am prescribed a medication that is excluded from coverage?
If you are prescribed a medication that is not covered, like certain brand-name drugs, it may have a generic alternative available. It's important to discuss with your doctor if a suitable alternative can be prescribed that is covered under your plan, ensuring you have access to necessary treatment.
Who can I contact for help with the PA process?
If you have questions or need assistance with the prior authorization process, calling the customer service number on your insurance card is recommended. Additionally, your healthcare provider's office can help navigate the requirements for submitting the PA request on your behalf.
Common mistakes
Filling out the Prime Therapeutics PA form can be tricky, and mistakes can lead to delays or denials in receiving necessary medications. One common mistake is not providing complete patient information. It’s important to ensure that all personal details are accurate and comprehensive. Missing out on critical information can stall the approval process.
Another mistake is failing to include the correct medication details. Make sure to list the exact name and dosage of the prescribed medication. Providing incorrect or incomplete medication information can result in a denial. Review the medication against the recommended list carefully.
Choosing the wrong format when submitting supporting documents is also a common error. If the form requests specific documents, ensure that you submit them in the required format. Submitting files in an unapproved format could lead to the rejection of your request.
Many people overlook signature requirements. When you fill out the form, ensure that all required signatures are included. An unsigned form can stall the process significantly. It’s a simple step, yet it’s crucial for timely processing.
Inaccurate or vague medical necessity statements can cause problems too. Be specific when detailing why the medication is necessary. Clear, precise language provides a better chance for approval, as vague statements may not satisfy the reviewer’s requirements.
Another common error is not checking for updates. The medication lists and covered alternatives can change. Always verify you are working with the most current information before submitting your request. Sticking to outdated drugs or procedures can lead to denials.
People often forget to double-check eligibility for specific programs. Ensure the patient is enrolled in the right plan that covers the requested medication. Even if all documentation is correct, incorrect eligibility can derail the process.
When filing out the form, some might assume that additional documentation won’t be necessary. However, various cases may require extra medical records or notes from the prescribing physician. Providing this information upfront can reduce back-and-forth communication and speed up approval.
Talking with the pharmacy is also essential. Some individuals neglect to coordinate with their pharmacy to confirm that it is part of the network and can fill the prescription. Making sure your pharmacy understands the authorization requirements can save time in the long run.
Lastly, people often misunderstand the timeframe for approvals. It is crucial to keep in mind that responses may take time, and following up is key. Failing to follow up can cause unnecessary delays if your request has been overlooked. Keeping track of your submission and following through ensures that you’ll get the care you need without undue delay.
Documents used along the form
Along with the Prime Therapeutics Prior Authorization (PA) form, several other forms and documents are often used in conjunction with it. Each serves a specific purpose in the prior authorization process and managing pharmacy benefits. The following is a list of these related documents.
- Drug Prior Authorization Request Form: This form is used to request authorization from the insurance provider for specific medications that may not be covered without prior approval.
- Quantity Limit Prior Authorization Form: This document is necessary when a prescribed medication exceeds the standard quantity limits set by the insurance provider.
- Responsible Steps Program Forms: These forms facilitate the request for medications under the Responsible Steps program, which may involve step therapy requirements.
- Pharmacy Exclusion Lists: This document provides a list of medications that are excluded from coverage, informing patients of their options regarding generics or alternatives.
- Patient Authorization Form: This form allows healthcare providers to obtain permission from the patient to share medical information with the insurance company for processing claims.
- Clinical Documentation Guidelines: Guidelines detailing the necessary medical records or evidence that must accompany a PA request for certain prescriptions.
- Specialty Pharmacy Enrollment Form: This document is filled out to enroll patients in specialty pharmacy services, required for certain high-cost medications.
Understanding these forms and documents is important for navigating the prior authorization process effectively. Accurate completion and timely submission can help ensure patients receive the medications they need without unnecessary delays.
Similar forms
The Prime Therapeutics Prior Authorization (PA) form is similar to several other documents used in the healthcare industry. Each of these documents serves to ensure appropriate use of medications and related services, guiding healthcare providers and patients through the necessary processes. Here’s a breakdown of these similar documents:
- Prior Authorization Form: This form, used by various healthcare plans, requires healthcare providers to obtain approval for specific medications before they can be dispensed. Like the Prime Therapeutics form, it ensures that the prescribed treatments meet medical necessity criteria.
- Medication Request Form: Often used for specialty medications, this form must be filled out by a healthcare provider when requesting coverage for certain drugs that may not be routinely covered. Similar to the Prime Therapeutics PA form, this document collects pertinent patient information and medical justification.
- Step Therapy Form: This document outlines a protocol that requires patients to try less expensive or lower-step medications before advancing to more costly options. Like the Prime Therapeutics form, it aims to promote cost-effective medication use and optimize patient care.
- Quantity Limit Form: This form is used to request exceptions to established medication quantity limits. It parallels the Prime Therapeutics PA form by requiring documentation of medical necessity when a patient needs more than the set limit for a specific medication.
- Clinical Prior Authorization Form: Used in various health plans, this form helps ensure that the clinical guidelines are met before certain treatments are approved. It is akin to the Prime Therapeutics form in that it emphasizes adherence to established medical evidence and protocols.
- Pharmacy Benefit Management (PBM) Request Form: This form is utilized by PBMs to manage and monitor drug benefits. Like the Prime Therapeutics PA form, it is focused on appropriate medication use and compliance with insurance policies.
Dos and Don'ts
When filling out the Prime Therapeutics PA form, follow these guidelines to ensure a smooth process:
- Do: Provide clear and accurate patient information. Make sure to include the patient's full name and insurance details.
- Do: Check all medication requirements. Be aware of which medications require prior authorization.
- Do: Attach necessary medical documentation. Include any relevant records that support the need for the requested medication.
- Do: Use the most current forms. Always download and fill out the latest version of the PA form.
- Don't: Leave any fields blank. Every section of the form should be filled out completely.
- Don't: Forget to sign and date the form. An unsigned form will likely delay the approval process.
- Don't: Ignore submission deadlines. Ensure you submit the form within the required time frame to avoid issues with coverage.
Misconceptions
Understanding the Prime Therapeutics Prior Authorization (PA) form can be challenging, and several misconceptions can lead to confusion. Here’s a breakdown of commonly held myths and the truths about the PA process related to Prime Therapeutics:
- Misconception 1: Only new patients need prior authorization.
- Misconception 2: All medications require prior authorization.
- Misconception 3: Prior authorization slows down the process to receive medications.
- Misconception 4: Patients cannot appeal a prior authorization denial.
- Misconception 5: All insurance plans require prior authorization for the same medications.
- Misconception 6: All prescriptions covered by the plan are automatically approved.
- Misconception 7: Generics do not require prior authorization.
- Misconception 8: Submitting a prior authorization request guarantees approval.
- Misconception 9: Once approved, there's no need to consider the quantity limits.
- Misconception 10: The PA process is the same for all drugs.
This is not true. While new prescriptions after a specific date may require approval, patients already on therapy can often continue their medications without interruption.
Only certain medications, as listed by the provider, require prior authorization. Most prescriptions can be filled without this process.
While there may be some delay, prior authorization is designed to ensure patients receive medically appropriate therapies based on clinical guidelines.
Patients do have the option to appeal denials. If a request is denied, providers and patients can work together to submit additional documentation for reconsideration.
Different insurance plans may have varying requirements. Prior authorization will depend on the specific policy and the medications involved.
While many prescriptions are covered, prior authorization is necessary for certain drugs to evaluate their medical necessity based on guidelines.
This isn't universally true. Some generic medications may also fall under the prior authorization requirement based on specific drug classifications.
Approval is not guaranteed. Each request is evaluated based on specific criteria, and additional documentation may be needed to support the request.
Quantity limits may still apply. Patients may need to work with their healthcare providers to ensure prescriptions fit within those limits or to request exceptions.
This is misleading. Different drugs may have unique prior authorization requirements, deadlines, and processes based on classifications and health guidelines.
Being informed about these misconceptions can help members navigate their healthcare benefits more effectively. Always reach out to your healthcare provider or insurance representative for clarification tailored to your situation.
Key takeaways
When using the Prime Therapeutics Prior Authorization (PA) form, understanding the requirements and processes can facilitate obtaining necessary medication. Below are key takeaways that can help guide you through the completion and use of the form.
- Eligibility Criteria: Prior authorization is required for specific oral oncology medications. Ensure that you verify the list to confirm if the prescribed medication needs prior authorization.
- Patient-Specific Documentation: Submitting the PA form necessitates including detailed patient information. This documentation should support the request and illustrate medical necessity.
- Continuity of Care: Patients already on therapy with the specified medications can continue their prescriptions without interruption, provided there is consistent treatment documented.
- Review Process: The request will be evaluated based on product labeling and clinical guidelines. It’s important to align the treatment indication with accepted standards.
- Specialty Pharmacy Coordination: If the medication falls under the specialty category, be prepared to work closely with the preferred specialty pharmacy, which is CVS Caremark. Their contact number is also helpful if issues arise.
- Responsible Quantity Program: Be attentive to the limits placed on medication quantities. The Responsible Quantity program regulates the maximum amounts for certain drugs based on FDA guidelines.
- Adjustments for Higher Quantities: If a larger quantity is necessary, your healthcare provider can submit a specific request for authorization to cover the additional amount, ensuring a seamless process for patients needing more medication.
- Updates on Coverage: Keep informed about changes in medication coverage. Some brand-name drugs may no longer be covered, but there could be generic alternatives available. Always check the latest list for updates.
These takeaways serve as a comprehensive guide for successfully navigating the Prime Therapeutics Prior Authorization form. By adhering to these points, you can ensure a smoother experience in obtaining medication through the pharmacy benefit program.
Browse Other Templates
What Is a Ddq - This form ensures compliance with California’s legal requirements for service.
Tronox Trust Payout 2022 - This claim form is essential for recovering costs related to injuries caused by Tronox.
