Fill Out Your Stoichiometry Worksheet Form
The Stoichiometry Worksheet serves as a vital educational tool for students seeking to grasp the concepts of chemical reactions and mole calculations in a structured manner. Through a series of thoughtfully designed exercises, the worksheet encourages users to explore the relationships between reactants and products in chemical equations. Typical tasks include calculating the moles of different substances formed or required in a reaction, allowing learners to apply their understanding of classic reactions such as the decomposition of potassium chlorate into potassium chloride and oxygen, as well as the combination of iron with oxygen to produce iron(III) oxide. Moreover, the worksheet encourages problem-solving by presenting questions of varying difficulty, including straightforward mole conversions and more challenging queries that require deeper analytical thinking. For instance, students are prompted to find not only the number of moles produced from given quantities but also to convert these moles into grams, thereby bridging the gap between theoretical calculations and practical laboratory application. The inclusion of varying examples, such as the combustion of butane, highlights real-world relevance, illustrating the utility of stoichiometry in both academic and practical contexts.
Stoichiometry Worksheet Example
Stoichiometry Worksheet and Key
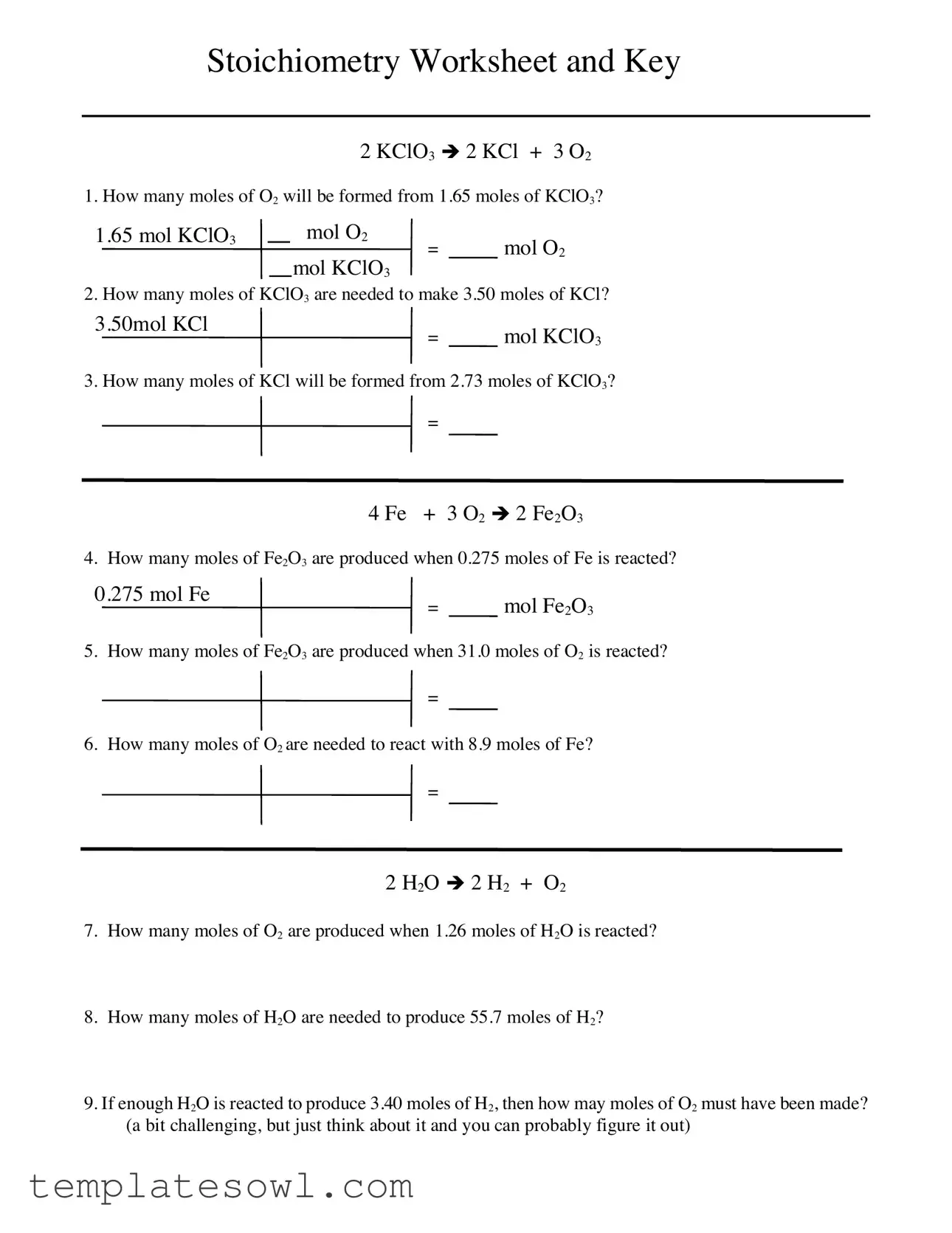
2 KClO3 è 2 KCl + 3 O2
1. How many moles of O2 will be formed from 1.65 moles of KClO3?
1.65 mol KClO3 |
mol O2 |
mol O2 |
|
= |
|
|
mol KClO3 |
|
2. How many moles of KClO3 are needed to make 3.50 moles of KCl?
3.50mol KCl
= mol KClO3
3. How many moles of KCl will be formed from 2.73 moles of KClO3?
=
4 Fe + 3 O2 è 2 Fe2O3
4. How many moles of Fe2O3 are produced when 0.275 moles of Fe is reacted?
0.275 mol Fe
= mol Fe2O3
5. How many moles of Fe2O3 are produced when 31.0 moles of O2 is reacted?
=
6. How many moles of O2 are needed to react with 8.9 moles of Fe?
=
2H2O è 2 H2 + O2
7.How many moles of O2 are produced when 1.26 moles of H2O is reacted?
8.How many moles of H2O are needed to produce 55.7 moles of H2?
9.If enough H2O is reacted to produce 3.40 moles of H2, then how may moles of O2 must have been made? (a bit challenging, but just think about it and you can probably figure it out)
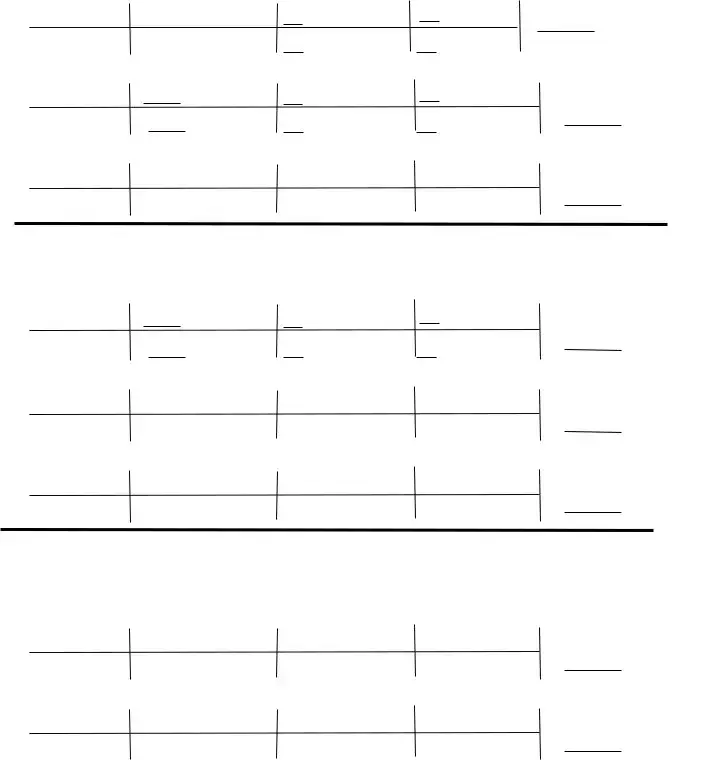
2 KClO3 è 2 KCl + 3 O2
10. How many grams of O2 will be formed from 3.76 grams of KClO3? |
|
||
3.76g KClO3 1 mol KClO3 |
mol O2 |
g O2 = |
g O2 |
122.55 g KClO3 |
mol KClO3 |
mol O2 |
|
11. How many grams of KClO3 are needed to make 30.0 grams of KCl?
30.0 g KCl |
mol KCl |
mol KClO3 |
g KClO3 |
= |
|
g KCl |
mol KCl |
mol KClO3 |
|
|
|
12.How many grams of KCl will be formed from 2.73 g of KClO3?
2.73g KClO3
=
4 Fe + 3 O2 è 2 Fe2O3
13. How many grams of Fe2O3 are produced when 42.7 grams of Fe is reacted?
42.7 g Fe |
mol Fe |
mol Fe2O3 |
g Fe2O3 |
|
g Fe |
mol Fe |
= |
|
mol Fe2O3 |
14. How many grams of Fe2O3 are produced when 17.0 grams of O2 is reacted?
17.0 g O2 |
= |
|
15. How many grams of O2 are needed to react with 125 grams of Fe?
=
Some cars can use butane (C4H10) as fuel:
|
2 C4H10 + 13 O2 è 8 CO2 + 10 H2O |
|
16. |
How many grams of CO2 are produced from the combustion of 100. grams of butane? |
|
100. g C4H10 |
= |
|
|
|
|
17. |
How many grams of O2 are needed to react with of 100. grams of butane? |
|
100. g C4H10 |
= |
|
|
|
|
18 How many grams of H2O are produced when 5.38g of O2 is reacted? |
|
|
g KClO3
g KCl
g Fe2O3
g Fe2O3
g CO2
g O2
KEY
è
1. How many moles of O2 will be formed from 1.65 moles of KClO3?
1.65 mol KClO3 |
3 mol O2 |
2.48 mol O2 |
|
= |
|
|
2 mol KClO3 |
|
2. How many moles of KClO3 are needed to make 3.50 moles of KCl?
3.50 mol KCl |
2 mol KClO3 |
mol KClO3 |
|
= 3.50 |
|
|
2 mol KCl |
|
3. How many moles of KCl will be formed from 2.73 moles of KClO3?
2.73 moles KClO3 |
2 mol KCl |
2.73 mol KCl |
|
= |
|
|
2 mol KClO3 |
|
|
4 Fe + 3 O2 è 2 Fe2O3 |
|
4. How many moles of Fe2O3 are produced when 0.275 moles of Fe are reacted?
0.275 mol Fe |
2 mol Fe2O3 |
= |
0.138 mol Fe2O3 |
|
4 mol Fe |
||
|
|
|
5. How many moles of Fe2O3 are produced when 31.0 moles of O2 are reacted?
31.0 mol O2 |
2 mol Fe2O3 |
= 20.7 mol Fe2O3 |
|
3 mol O2 |
|
|
|
6. How many moles of O2 are needed to react with 8.9 moles of Fe?
8.9 mol Fe |
3 mol O2 |
= |
6.7 |
mol O2 |
|
4 mol Fe |
|||
|
|
|
|
2H2O è 2 H2 + O2
7.How many moles of O2 are produced when 1.26 moles of H2O is reacted?
1.26mol H2O 1 mol O2= .630 mol O2 2 mol H2O
8.How many moles of H2O are needed to produce 55.7 moles of H2?
55.7mol H2 2 mol H2O = 55.7 mol H2O 2 mol H2
9.If enough H2O is reacted to produce 3.40 moles of H2, then how may moles of O2 must have been made? (a bit challenging, but just think about it and you can probably figure it out)
3.40 mol H2 1 mol O2 |
1.70 |
mol O2 |
= |
||
2 mol H2 |
|
|
2 KClO3 è 2 KCl + 3 O2
10. How many grams of O2 will be formed from 3.76 grams of KClO3? |
|
|
||||
3.76g KClO3 |
1 mol KClO3 |
3 |
mol O2 |
32.00 g O2 |
= |
1.47 g O2 |
|
122.55 g KClO3 |
2 mol KClO3 |
1 mol O2 |
|
|
|
11. How many grams of KClO3 are needed to make 30.0 grams of KCl? |
|
|
||||
30.0 g KCl |
1 mol KCl |
2 |
mol KClO3 |
122.55 g KClO3 |
= 49.3 g KClO3 |
|
|
74.55 g KCl |
2 |
mol KCl |
1 mol KClO3 |
|
|
12. How many grams of KCl will be formed from 2.73 g of KClO3?
2.73 g KClO3 1 mol KCl O3 |
2 |
mol KCl |
74.55 g |
= 1.66 g KCl |
122.55 g KClO3 |
2 |
mol KCl O3 |
1 mol KCl |
4 Fe + 3 O2 è 2 Fe2O3
13. How many grams of Fe2O3 are produced when 42.7 grams of Fe is reacted?
42.7 g Fe |
1 |
mole Fe |
2 |
mol Fe2O3 |
159.70 g Fe2O3 |
= |
61.0 g Fe2O3 |
|
|
55.85 |
g Fe |
4 |
mol Fe |
1 mol Fe2O3 |
|||
|
|
|
||||||
14. How many grams of Fe2O3 are produced when 17.0 grams of O2 is reacted? |
= |
56.6 g Fe2O3 |
||||||
17.0 g O2 |
1 mol O2 |
2 mol Fe2O3 |
159.70 |
g Fe2O3 |
||||
|
|
|
|
|
|
|
|
|
|
32.00 |
g O2 |
3 |
mol O2 |
1 mol Fe2O3 |
|
|
|
15. How many grams of O2 are needed to react with 125 grams of Fe?
125 g Fe |
1 mol Fe |
3 mol O2 |
32.00 g O2 |
= |
53.7 |
g O2 |
||
|
55.85 |
g Fe |
4 |
mol Fe |
1 mol O2 |
|||
|
|
g |
|
|||||
|
|
|
|
|
||||
Some cars can use butane (C4H10) as fuel: |
|
|
|
|
|
|
||
|
|
2 C4H10 + 13 O2 è 8 CO2 + 10 H2O |
|
|
|
|||
16. How many grams of CO2 are produced from the combustion of 100. grams of butane? |
|
|||||||
100. g C4H10 |
1 mol C4H10 |
8 mol CO2 |
44.01 g CO2 |
= |
303 |
g CO2 |
||
|
58.14 g C4H10 |
2 mol C4H10 |
1 mol CO2 |
|||||
|
|
|
|
|||||
17. How many grams of O2 are needed to react with of 100. grams of butane?
100. g C4H10 |
1 mol C4H10 |
13 mol O2 |
32.00 g O2 |
= |
358 |
g O2 |
|
58.14 g C4H10 |
2 mol C4H10 |
1 mol O2 |
|||
|
|
|
|
|||
18 How many grams of H2O are produced when 5.38g of O2 is reacted? |
|
|
|
|||
5.38g O2 |
1 mol O2 |
10 mol H2O |
18.02 g H2O |
= |
2.33 |
g H2O |
|
32.00 g O2 |
13 mol O2 |
1 mol H2O |
|||
|
|
|
|
|||
Form Characteristics
| Fact Name | Description |
|---|---|
| Stoichiometric Relationships | The worksheet outlines several stoichiometric calculations based on chemical equations, demonstrating how to convert between moles, grams, and chemical species. |
| Mole Conversions | It provides specific questions regarding the conversion of moles of one reactant to moles of products or other reactants, allowing practice with mole ratio understanding. |
| Example Reactions | Included are various chemical reactions, such as the decomposition of potassium chlorate (KClO3) and the combustion of butane (C4H10), which illustrate the practical application of stoichiometry. |
| Grams to Moles Calculations | The worksheet features exercises that require converting grams of a substance to moles, reinforcing the connection of mass and number of particles in a chemical reaction. |
Guidelines on Utilizing Stoichiometry Worksheet
Completing the Stoichiometry Worksheet is an essential step towards grasping key concepts in chemistry. By working through the exercises, you will deepen your understanding of chemical reactions and the relationships between different substances. Follow these steps to fill out the worksheet accurately.
- Begin by carefully reading each question on the worksheet to understand the relation between the substances involved.
- For each question, identify the known quantities (like moles or grams) and the desired quantities you need to find.
- Refer to the balanced chemical equations provided in the worksheet to determine the correct molar ratios between the substances.
- For questions asking for moles, set up the equation using appropriate conversions based on these ratios.
- If the question involves grams, remember to convert grams to moles using the molar mass of the compound—it's essential to have accurate molar masses handy.
- Once you have computed the values, make sure to double-check your calculations for accuracy.
- Record your answers clearly in the spaces provided on the worksheet.
- Review your completed worksheet to ensure all questions are answered and calculations are correct.
What You Should Know About This Form
What is the purpose of the Stoichiometry Worksheet?
The Stoichiometry Worksheet is designed to help students practice calculations involving chemical reactions. It provides a structured approach to understanding how reactants and products relate to one another based on the balanced equations. By working through the problems, learners can build their skills in converting between moles, grams, and understanding reaction yields.
How do I determine the number of moles of a product from a given number of moles of a reactant?
To find out how many moles of a product are produced from a certain amount of a reactant, use the coefficients from the balanced chemical equation. For example, if the equation shows that 2 moles of the reactant yield 3 moles of product, you can set up a proportion. Multiply the number of moles of the reactant by the ratio of the product's coefficient to the reactant's coefficient.
Are the calculations based on real chemical reactions?
Yes, the calculations included in the worksheet are based on actual chemical equations. These represent common reactions, such as the decomposition of potassium chlorate (KClO3) and the combustion of butane. Understanding these reactions can provide insight into practical applications in chemistry and everyday life.
Can I complete the worksheet without prior knowledge of stoichiometry?
While prior knowledge of stoichiometry is helpful, the worksheet is designed to guide students through the necessary calculations step by step. By applying the concepts presented in the problems and utilizing the balanced equations, individuals can learn and complete the exercises effectively.
What do I do if I encounter a difficult question?
If you come across a challenging question, take a moment to break it down. Identify what you know and what you need to find. Reviewing the related chemical equation and considering similar examples may also provide clarity. Don't hesitate to reach out to a teacher or peer for assistance if you're still struggling.
What tools or materials should I have when completing this worksheet?
A calculator is essential for performing mathematical computations. It’s also helpful to have periodic tables available to reference molar masses. A notebook for rough work may assist you as you calculate and re-check your values.
How can I check my answers once I've completed the worksheet?
The worksheet often includes an answer key that provides the correct solutions for each question. Cross-referencing your results will help confirm your calculations are accurate. If any answers differ, reviewing your steps can help identify where a mistake may have occurred.
Is there a limit to how many times I can practice this worksheet?
No, there is no limit to how often you can use the Stoichiometry Worksheet. Repetition can enhance understanding, so feel free to retry the problems to reinforce your learning. Each attempt offers an opportunity to improve your skills and gain confidence.
What concepts should I understand before attempting the worksheet?
Before tackling this worksheet, it's beneficial to have a basic understanding of moles, molar mass, and the concept of balanced chemical equations. Familiarity with converting between grams and moles and using ratios from the equations will greatly aid in solving the problems presented.
Common mistakes
Filling out the Stoichiometry Worksheet can be challenging, and several common mistakes often occur during this process. First, one frequent error is neglecting to balance the chemical equations before attempting to solve the problems. When the equations are not balanced, the stoichiometric relationships among the reactants and products can lead to inaccurate mole calculations.
Another mistake people commonly make is failing to pay attention to the units associated with each measurement. For example, when converting grams to moles, it is crucial to use the correct molar mass of the substances involved. If someone miscalculates the molar mass or forgets to carry it throughout their calculations, the final answer will be compromised.
Misinterpreting the coefficients in chemical equations can also result in significant errors. It's essential to understand that coefficients indicate the ratio of moles between reactants and products. Some individuals may overlook these coefficients altogether, which can lead to erroneous mole ratios when solving for unknowns.
Precision in calculations is vital. Rounding too early in a multi-step calculation can lead to compounding errors. Instead, it is advisable to keep as many significant figures as possible until the final answer is calculated. This will yield a more accurate result and minimize discrepancies.
People also tend to confuse the concepts of moles and grams. It is not uncommon for someone to attempt to solve a problem in grams when the question specifically requests moles. Understanding when to convert between these units is crucial for proper problem-solving.
Another common issue arises when students fail to demonstrate their work or clearly label each step. This lack of organization can lead to confusion, making it difficult to identify mistakes. Clear documentation allows for easy tracing back through calculations to find where an error may have occurred.
In addition, some individuals might misapply the ideal gas law in calculations involving gases. Failing to recognize the conditions under which gas behaves ideally or neglecting the proper use of temperature and pressure can skew results significantly.
Finally, overlooking the final units of measurement can lead to conclusions that are inconsistent with the question asked. It is essential to ensure that the answer aligns not just quantitatively, but also qualitatively, with the question guidelines. Double-checking both calculations and units can prevent misunderstandings.
Documents used along the form
The Stoichiometry Worksheet is an important tool for students learning the concepts of chemical reactions and the relationships between reactants and products. Alongside it, several other documents and forms enhance the learning experience and support different aspects of chemical calculation. Here are some commonly used forms:
- Lab Report Template: This document provides a structured format for students to record their experiments, including objectives, methods, results, and conclusions.
- Data Analysis Sheet: This sheet is used to analyze collected data from experiments. It helps students organize their findings and draw necessary conclusions.
- Mole Conversion Worksheet: This worksheet focuses on converting between moles, grams, and molecules, assisting students in understanding these key concepts better.
- Balancing Chemical Equations Guide: This guide offers step-by-step instructions on how to balance various chemical equations, which is crucial in stoichiometry.
- Practice Quiz: A quiz that helps students test their understanding of stoichiometry concepts and chemical calculations, reinforcing their learning.
- Chemical Property Chart: This chart lists common chemicals and their properties, assisting students in recognizing patterns and relationships in reactions.
- Safety Data Sheet (SDS): Essential for lab safety, this document provides information on handling chemicals safely and what to do in case of emergencies.
Together, these forms and documents create a comprehensive system that helps students master stoichiometry and related chemical concepts effectively. Each resource complements the others to provide a well-rounded educational experience.
Similar forms
Laboratory Report Form: Like the Stoichiometry Worksheet, this document collects calculations and results related to chemical reactions. Both forms focus on quantifying products and reactants, helping to organize findings in a clear manner.
Balancing Chemical Equations Worksheet: This document emphasizes equating reactants and products in a reaction, similar to how the Stoichiometry Worksheet requires understanding and manipulating mole ratios derived from balanced equations.
Mole-to-Mass Conversion Activity: In both documents, the conversion between moles and mass is vital. The Stoichiometry Worksheet asks about mass in several questions, mirroring the core goal of this conversion activity.
Reaction Yield Calculation Sheet: Both forms focus on determining how much product is produced in a reaction and what quantities of reactants are necessary, aiding students in understanding yield and efficiency in chemical processes.
Stoichiometry Practice Problems: These practice sheets consist of questions similar to those in the Stoichiometry Worksheet. They challenge students to calculate moles, grams, and other quantities derived from chemical equations.
Chemical Reaction Worksheet: This document involves similar content by requiring individuals to analyze reactions, predict outcomes, and often perform stoichiometric calculations to achieve accuracy, thus complementing the Stoichiometry Worksheet.
Dos and Don'ts
When filling out the Stoichiometry Worksheet form, there are important guidelines to follow to ensure accuracy and clarity.
- Review the equations before starting to ensure you understand the reactions involved.
- Use clear and legible handwriting if writing by hand, or type if possible to avoid misinterpretation.
- Double-check your mole ratios to ensure they correspond correctly to the balanced chemical equations.
- Show all your work for each calculation to make it easier to follow your thought process.
- Label your units throughout to avoid confusion when converting between grams and moles.
- Avoid rushing through the questions; take your time to ensure accuracy.
- Don’t omit any calculations or steps, as this could lead to incomplete answers.
- Refrain from using calculators without understanding the formulas; this could create errors.
- Never ignore the significance of significant figures in your final answers, as this is crucial in scientific calculations.
By adhering to these guidelines, the likelihood of errors will diminish, significantly enhancing the quality of your work on the Stoichiometry Worksheet.
Misconceptions
The Stoichiometry Worksheet form is a valuable educational tool, but several misconceptions about it can lead to confusion. Understanding these misconceptions can improve learning and application of stoichiometric principles.
- Stoichiometry is just about balancing equations. While balancing equations is a crucial step, stoichiometry involves calculating amounts of reactants and products based on balanced reactions.
- All stoichiometry problems are the same. Each problem may require different approaches or conversion factors, contributing to a diverse range of calculations.
- Conversions are always in moles. While many calculations do utilize moles, some problems require conversions to grams or volume, depending on the context.
- Using the Stoichiometry Worksheet ensures correct answers. The worksheet provides a framework, yet comprehension and proper application of stoichiometric principles are essential for accuracy.
- Stoichiometry only applies to chemical reactions. Although it is primarily associated with chemical reactions, stoichiometry principles are applicable in other scientific fields, such as pharmacy and food science.
- All stoichiometry problems should have a single answer. Some problems may require multiple steps and calculations, leading to a final answer rather than a single direct calculation.
- Students can skip unit conversions if they know the numbers. Units are critical in stoichiometry. Failing to convert units can result in incorrect answers and misunderstandings.
- Understanding the Stoichiometry Worksheet means I understand stoichiometry. Mastery of stoichiometry requires a deeper understanding of concepts beyond simply filling out a worksheet.
- Once I learn stoichiometry, I’ll never forget it. This concept is complex, and regular practice is necessary to maintain proficiency.
- Stoichiometry doesn’t involve real-life applications. On the contrary, many industries, including pharmaceuticals and environmental science, rely heavily on stoichiometric calculations for practical applications.
Key takeaways
Understanding the Stoichiometry Worksheet is crucial for navigating chemical calculations effectively. Here are some key takeaways to assist you.
- The worksheet helps in calculating the number of moles in chemical reactions based on given equations.
- It provides a systematic approach to find the relationship between reactants and products.
- Writing down the balanced chemical equations accurately is essential before beginning calculations.
- Conversion factors, such as moles to grams, must be employed correctly to achieve the right results.
- Using molar ratios derived from balanced equations is necessary for accurate calculations of moles of products formed.
- Identify and label which quantities you are solving for; this will keep your calculations organized.
- Students should check their answers with the provided key to confirm their understanding and accuracy.
- Practice with various types of problems on the worksheet will enhance proficiency in stoichiometric calculations.
Browse Other Templates
Cd-516 - Accurate position descriptions are essential for effective job performance evaluations.
Post Office Forms - Ensure that the mailpiece you submit is opened and complete to meet the form's requirements.