Fill Out Your Student Chemical Equations Form
The Student Chemical Equations form is an essential tool for students exploring the fascinating world of chemistry. It provides a structured way to engage with key concepts such as chemical reactions and formulas. At the start of the form, students are required to fill in their name and date, setting the stage for the experiments to follow. The form includes important vocabulary, covering terms like reactants, products, moles, and conservation of matter, among others. Before diving into practical activities with the Gizmo™, students answer several prior knowledge questions, which helps assess their understanding of chemical principles. The activities guide students in balancing chemical equations, counting atoms, and practicing interpretation of formulas. Through hands-on interaction and guided questions, the form encourages critical thinking and application of knowledge. Ultimately, it fosters a deeper comprehension of how substances interact and transform in chemical processes, making learning about chemical equations engaging and impactful.
Student Chemical Equations Example
Name: ______________________________________ |
Date: ________________________ |
Student Exploration: Chemical Equations
Vocabulary: Avogadro’s number, chemical equation, chemical formula, chemical reaction, coefficient, combination, combustion, conservation of matter, decomposition, double replacement, molar mass, mole, molecule, product, reactant, single replacement, subscript
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
1.A candle is placed on one pan of a balance, and an equal weight is placed on the other pan. What would happen if you lit up the candle and waited for a while? ____________________
_________________________________________________________________________
2.Suppose the candle was placed in a large, sealed jar that allowed it to burn for several minutes before running out of oxygen. The candle and jar are balanced by an equal weight.
In this situation, what would happen if you lit up the candle and waited? ________________
_________________________________________________________________________
Gizmo
Burning is an example of a chemical reaction. The law of conservation of matter states that no atoms are created or destroyed in a chemical reaction. Therefore, a balanced chemical equation will show the same number of each type of atom on each side of the equation.
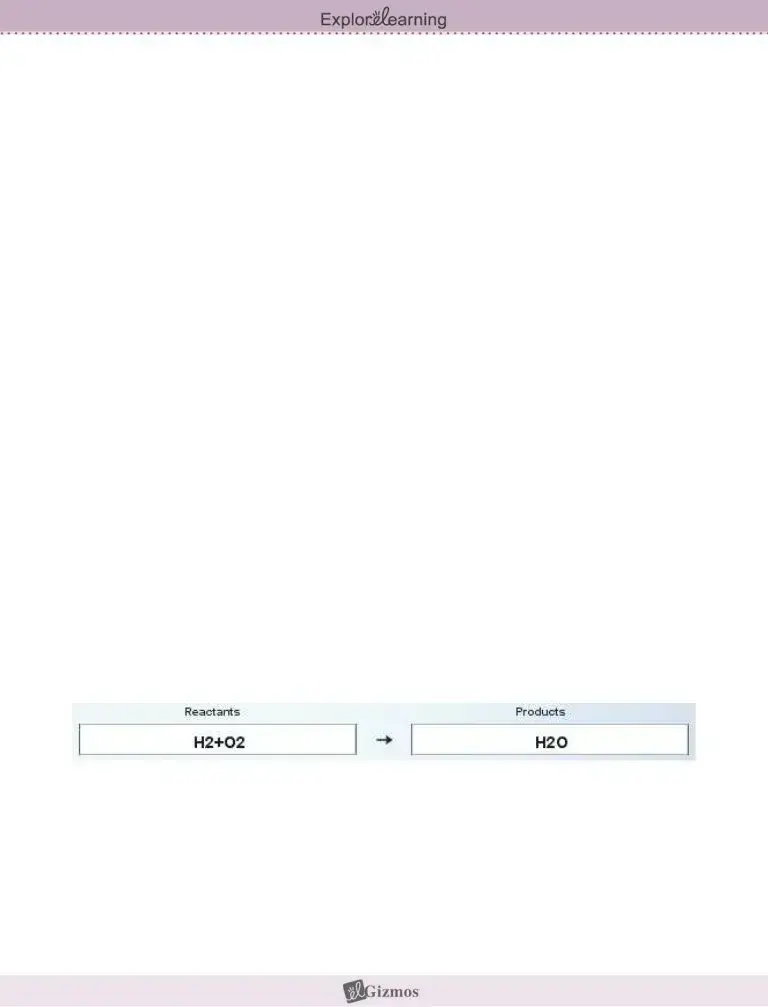
To set up an equation in the Chemical Equations Gizmo™, type the chemical formulas into the text boxes of the Gizmo. First, type in “H2+O2” in the Reactants box and “H2O” in the Products box. This represents the reaction of hydrogen and oxygen gas to form water.
1. Check that the Visual display is chosen on each side of the Gizmo, and count the atoms.
A. How many hydrogen atoms are on the Reactants side? ____ Products side? ____
B. How many oxygen atoms are on the Reactants side? ____ Products side? ____
2. Based on what you see, is this equation currently balanced? _________________________

Activity A: |
Get the Gizmo ready: |
|
|
Interpreting |
• |
Erase the chemical formulas in each text box. |
|
chemical formulas |
• |
Check that the Visual displays are selected. |
|
|
|
|
|
Introduction: To balance a chemical equation, you first need to be able to count how many atoms of each element are on each side of the equation. In this activity, you will practice counting the atoms that are represented in chemical formulas.
Question: How do we read chemical formulas?
1.Observe: Type “H2” into the Reactants box and hit Enter on your keyboard. Note that the formula is shown as H2 below. The small “2” in H2 is a subscript.
A.What does the “2” in H2 represent? _______________________________________
B.In general, what do you think a subscript in a chemical formula tells you? _________
___________________________________________________________________
C.Try typing in other subscripts next to the H, such as 3, 4, and 5. Is your answer to question B still true? Explain. ____________________________________________
2.Count: Clear the Reactants box, and type in a more complex chemical formula: “Ca(OH)2.” Look at the number of atoms shown.
A. How many of each type of atom do you see? Ca: _____ O: _____ H: _____
B.In general, what happens when a subscript is found outside of parentheses?
___________________________________________________________________
C.Try typing in other subscripts next to the (OH), such as 3, 4, and 5. Is your answer to question B still true? Explain. ____________________________________________
3.Practice: For each of the real chemical formulas below, calculate how many of each element there are. Check your answers for the first three formulas using the Gizmo.
AgCl3Cu2 |
Ag: _____ |
Cl: _____ |
Cu: _____ |
|
Ba(AsO4)2 |
Ba: _____ |
As: _____ |
O: _____ |
|
(NH4)3PO4 |
N: _____ |
H: _____ |
P: _____ |
O: _____ |
MnPb8(Si2O7)3 |
Mn: _____ |
Pb: _____ |
Si: _____ |
O: _____ |
Activity B:
Balancing equations
Get the Gizmo ready:
•Erase the chemical formulas in each text box.
Introduction: In a chemical reaction, the reactants are the substances that enter into the reaction, and the products are the substances that are made in the reaction. A chemical reaction is balanced if the numbers of reactant atoms match the numbers of product atoms.
Goal: Learn to balance any chemical equation.
1.Observe: To model how hydrogen and oxygen react to make water, type “H2+O2” into the Reactants box and “H2O” into the Products box.
As the equation is written, which element is not in balance? ________________________
Explain: _________________________________________________________________
2.Balance: To balance a chemical equation, you are not allowed to change the chemical formulas of the substances involved in the reaction. You are allowed to change the number of molecules of each substance by adding coefficients in front of the formulas.
A.To balance the oxygen atoms, add a “2” in front of the “H2O” in the Products box. How many oxygen atoms are found on each side of the equation now? _________
B.To balance the hydrogen atoms, add a “2” in front of the “H2” in the Reactants box. How many hydrogen atoms are found on each side of the equation now? _________
C.Is this equation currently balanced? _________ Click Show if balanced to check.
3.Apply: Now enter a more complex chemical reaction: Ca(OH)2 + HBr CaBr2 + H2O. List the numbers of each element in the tables below:
Ca
Reactants
OH
Br
Ca
Products
OH
Br
A.Which elements are out of balance? ______________________________________
B.Add coefficients to balance first the bromine (Br) and then the hydrogen (H) atoms. When the equation is balanced, write the complete formula below:
___________________________________________________________________
(Activity B continued on next page)

Activity B (continued from previous page)
4.Practice: Chemical reactions are generally classified into five groups, defined below. Balance each equation, using the Gizmo for help.
Combination (or synthesis) – two or more elements combine to form a compound.
• Na + O2 Na2O |
_________________________________________ |
||
• La2O3 + H2O La(OH)3 |
_________________________________________ |
||
• N2O5 + H2O HNO3 |
_________________________________________ |
||
|
|
||
Decomposition |
– a compound breaks down into elements and/or simpler compounds. |
||
• KNO3 KNO2 + O2 |
_________________________________________ |
||
• NaN3 Na + N2 |
_________________________________________ |
||
• NH4NO3 N2O + H2O |
_________________________________________ |
||
|
|||
Combustion |
– a fuel reacts with oxygen to release carbon dioxide, water, and heat. |
||
• CH4 + O2 CO2 + H2O |
_________________________________________ |
||
• C3H8 + O2 CO2 + H2O |
_________________________________________ |
||
•C6H12O6 + O2 CO2 + H2O _________________________________________
Single replacement – an element replaces another element in a compound.
• |
KCl + F2 KF + Cl2 |
_________________________________________ |
||
• |
Mg + HCl MgCl2 + H2 |
_________________________________________ |
||
• |
Cu + AgNO3 Cu(NO3)2 + Ag |
_________________________________________ |
||
|
||||
Double replacement |
– two compounds switch parts with one another. |
|||
• |
AgNO3 + K2SO4 Ag2SO4 + KNO3 |
___________________________________ |
||
• |
Mg(OH)2 + HCl MgCl2 + H2O |
|
___________________________________ |
|
•Al(OH)3 + H2SO4 Al2(SO4)3 + H2O ___________________________________

Activity C: Molar mass
Get the Gizmo ready:
•Erase the chemical formulas in each text box.
•In the middle menu, select Molar mass.
Introduction: Chemists are often interested in obtaining a certain amount of product from a chemical reaction. But how is this done? To calculate the proportions of reactants needed to form a desired product, it is necessary to understand a unit of quantity called the mole.
Question: How do chemists know how much of each substance to mix?
1.Observe: The masses of atoms and molecules are measured in universal mass units (u). A universal mass unit is approximately the mass of a proton. Hydrogen gas has a molecular mass of 2.0158 u.
A.Type the formula “H2” into the Reactants box. What is the molar mass of hydrogen gas, H2? ________________________
B.What is the relationship between the molecular mass and the molar mass of a substance? _________________________________________________________
The molar mass of a substance is the mass of one mole of the substance. There are 6.0221415 × 1023 molecules (or atoms) of a substance in one mole. (This value is called Avogadro’s number.)
2.Gather data: The balanced equation to synthesize water is: 2H2 + O2 2H2O. Use the Gizmo to find the molar masses of each substance in this equation:
2H2 __________ |
O2 __________ |
2H2O __________ |
3.Analyze: Based on the molar masses, how can you tell that an equation is balanced?
_________________________________________________________________________
_________________________________________________________________________
4.Challenge yourself: Suppose you wanted to make 100 grams of water. How much hydrogen and oxygen would you need to make 100 grams of water with nothing left over? Explain your answer.
Hydrogen: __________Oxygen: __________
_________________________________________________________________________
_________________________________________________________________________
_________________________________________________________________________
Form Characteristics
| Fact Name | Description |
|---|---|
| Name Required | The Student Chemical Equations form requires the student's name to identify ownership and participation. |
| Date Included | The date field is essential for documenting when the form was completed, adding context to the student's work. |
| Vocabulary List | A comprehensive list of terms is provided, relevant to chemical equations, aiding in vocabulary building and reinforcing understanding. |
| Prior Knowledge Questions | Students are asked questions that gauge their understanding of basic principles before they engage with the learning tools. |
| Conservation of Matter | The form emphasizes the law of conservation of matter, a key principle that explains atoms are neither created nor destroyed in a reaction. |
| Balancing Equations Exercise | Activities included assist students in learning how to balance chemical equations, fostering essential problem-solving skills needed in chemistry. |
| Molar Mass Calculation | Students calculate the molar mass of substances as part of their learning, linking concepts of mass and quantity in chemical reactions. |
Guidelines on Utilizing Student Chemical Equations
Once you've gathered your materials and understood the context of the Student Chemical Equations form, it's time to fill it out accurately. Start by providing your personal information followed by responding to the questions about prior knowledge. Practice and analyze the equations as detailed in the activities outlined in the form. This process will help solidify your understanding of chemical equations and reactions.
- Write your Name in the designated space.
- Fill in the Date of when you are completing the form.
- In the section titled Prior Knowledge Questions, answer the two questions provided based on your understanding of what occurs when the candle is burned.
- Access the Gizmo and ensure the Visual display is selected on both sides of the Gizmo.
- Complete the warm-up activity by typing “H2 + O2” in the Reactants box and “H2O” in the Products box. Count the number of atoms as instructed.
- Proceed to Activity A and erase any formulas in the text boxes. Type "H2," observe its representation, and answer the questions about subscripts.
- Clear the Reactants box again, and type "Ca(OH)2" to count the atoms of each type.
- Complete the remaining questions about counting atoms for the provided real chemical formulas.
- For Activity B, erase the formulas and try to balance the equations as presented. Record the numbers of each element in the tables provided.
- In Activity C, erase any formulas again and select Molar mass from the middle menu. Start with hydrogen gas by typing “H2” and answer the questions that follow.
- Gather data on the molar masses for the balanced water synthesis equation and analyze the results.
- Conclude by answering the challenge question about producing a specific mass of water using the correct amounts of hydrogen and oxygen.
What You Should Know About This Form
What is the purpose of the Student Chemical Equations form?
The Student Chemical Equations form is designed to help students understand and practice chemical equations through a structured activity. It guides them in recognizing reactants, products, and how to balance chemical equations, enhancing their grasp of chemical reactions and conservation of matter.
What prior knowledge should students have before using the Gizmo?
Before using the Gizmo, students are encouraged to think critically about basic concepts such as the nature of a candle burning and its effects in different environments. Questions regarding the balance of weight and oxygen availability help set the stage for understanding how reactions work, particularly in recognizing that matter is not created or destroyed during a chemical reaction.
What is the significance of balancing chemical equations?
Balancing chemical equations is vital because it reflects the law of conservation of matter. This principle states that the same number of atoms must exist on both sides of the equation. Balancing ensures accurate representation of a reaction, providing insight into the amounts of reactants and products involved.
How do students use subscripts in chemical formulas?
Students learn that subscripts in chemical formulas indicate the number of atoms of each element in a molecule. For example, "H2" signifies two hydrogen atoms. They are encouraged to explore the meaning of subscripts in chemical formulas further by experimenting with different numbers and analyzing resulting changes in the formulas' representation.
What are the different types of chemical reactions students will explore?
Students will explore five types of chemical reactions: combination, decomposition, combustion, single replacement, and double replacement. Each type has unique characteristics that describe how reactants interact and transform into products. Understanding these categories aids students in predicting the outcomes of reactions.
What is Avogadro's number, and why is it important?
Avogadro's number, approximately 6.022 × 1023, is the number of molecules in one mole of a substance. This number is fundamental in chemistry because it bridges the gap between atomic scale measurements and macroscopic quantities. It allows chemists to calculate the amount of substances needed for reactions accurately.
How can students analyze and gather data on molar mass?
In the activity, students gather data on molar mass by inputting chemical formulas into the Gizmo. The tool provides the molar mass of each substance, which helps them understand the relationship between molecular mass and practical quantities necessary for reactions. Analyzing this data improves their comprehension of how to measure and balance effectively.
What should students do if they want to make a specific mass of a product?
If students aim to synthesize a specific mass of a product, like 100 grams of water, they need to calculate how much of each reactant they require based on the balanced equation. This involves using stoichiometric relationships derived from molar masses and applying these to determine the necessary quantities of hydrogen and oxygen.
How does the Gizmo facilitate the learning process?
The Gizmo provides an interactive platform for students to visualize and experiment with chemical equations. Its user-friendly interface allows students to manipulate equations, observe outcomes in real time, and receive immediate feedback on their attempts to balance equations, thus reinforcing their learning experience.
Common mistakes
Filling out the Student Chemical Equations form correctly is crucial for understanding chemical reactions. One common mistake is failing to write the name and date. These basic details are essential for identification and organization.
Another frequent error involves skipping prior knowledge questions. Students often overlook these questions, yet they are vital for assessing understanding before beginning the Gizmo. Ignoring them can lead to a lack of comprehension of key concepts.
Many students struggle with typing chemical formulas accurately. A mistake here can lead to incorrect answers down the line. Always double-check spelling and formatting. For example, a single incorrect subscript can change the entire equation.
When counting atoms, students sometimes forget to tally both sides of the equation. This leads to an unbalanced equation, which is a fundamental error. Each side must have the same number of each atom.
Some users mistakenly believe they can change the chemical formulas to balance the equations. That’s incorrect. The formulas represent specific substances and cannot be altered. Only coefficients may be changed to achieve balance.
During the activity involving complex chemical formulas, it’s easy to miscount atoms when subscripts are involved. It is essential to clearly understand what subscripts signify. They indicate how many atoms of each element are present in a molecule.
Many students rush through the practice activity and do not check their answers using the Gizmo. Verifying answers is a crucial step that should never be skipped. It ensures that the information is accurate and enhances learning.
Students sometimes confuse the different types of chemical reactions. Misidentifying a reaction can lead to incorrect balancing attempts. Knowing the categories—like combustion or decomposition—is important for applying the correct methods.
Finally, some learners struggle with the concept of molar mass and its application in chemical equations. It’s easy to overlook these calculations, but they are fundamental for understanding the quantities in reactions. Always take the time to analyze and interpret molar mass correctly.
Documents used along the form
The Student Chemical Equations form is often accompanied by several other important documents that aid in understanding and conducting chemical experiments. These forms provide essential data, guidelines, and frameworks for interpreting chemical reactions effectively and ensuring that students gain a comprehensive grasp of the underlying concepts. Below is a list of documents frequently used alongside the Student Chemical Equations form, each explained briefly.
- Lab Safety Guidelines: This document outlines essential safety precautions that students must follow during their laboratory work. It includes information on personal protective equipment (PPE), how to handle chemicals safely, and emergency procedures in case of accidents.
- Preparation of Reagents: A guide that describes the procedures for preparing various chemical solutions and mixtures needed for experiments. It ensures that students understand concentrations and solution preparation methods.
- Chemical Reaction Observations Sheet: This form allows students to record qualitative and quantitative observations during the experiment. It helps in documenting the results and provides a basis for analysis later on.
- Equation Balancing Worksheet: A practice worksheet specifically designed to help students master the skill of balancing chemical equations. It includes examples and spaces for students to practice and self-assess their understanding.
- Safety Data Sheets (SDS): These provide detailed information on the hazards associated with specific chemicals used in the laboratory. SDS documents include information on toxicity, handling, storage, and disposal.
- Experimental Design Template: This document guides students in planning their experiments. It includes sections for hypotheses, variables, control measures, and procedures, encouraging thoughtful scientific inquiry.
- Post-Lab Questions: Questions related to the experiment that encourage critical thinking and reflection on the concepts learned. These questions help reinforce knowledge and assess understanding.
- Grade Evaluation Rubric: This rubric provides criteria for assessing students' laboratory reports and performances. It specifies expectations for organization, content accuracy, and analytical depth.
- Glossary of Chemical Terms: A handy reference that defines key terminologies used throughout chemistry courses. Students use this document to clarify concepts and reinforce their understanding of the language of chemistry.
These documents enrich the educational experience by providing structured support in various aspects of chemical experimentation and understanding. They enhance not just the procedural aspects of laboratory work but also encourage students to think critically about their findings and the safety protocols necessary in any scientific inquiry.
Similar forms
-
Lab Report Template: Similar to the Student Chemical Equations form, the lab report template includes sections for writing observations, methods used, and results obtained. Both documents require students to record their findings and analyze chemical reactions.
-
Chemistry Workbook: The chemistry workbook often contains exercises that explore chemical equations and concepts related to them. Much like the Student Chemical Equations form, it guides students through practicing their understanding of atoms, molecules, and how to balance equations.
-
Experiment Instruction Sheet: This document outlines the steps for conducting specific experiments, similar to the Student Chemical Equations form. Both provide clear guidelines for students, helping them understand the experimental setup, observation, and analysis process involved in chemical reactions.
-
Chemical Equation Worksheet: Like the Student Chemical Equations form, this worksheet focuses on writing and balancing chemical equations. Both documents encourage students to engage with key concepts and provide spaces for computation and explanations regarding reactants and products.
Dos and Don'ts
When filling out the Student Chemical Equations form, attention to detail can make all the difference. Here are five guidelines to help you navigate the process effectively:
- Do write clearly. Ensure that your handwriting is legible so your answers can be easily read.
- Don't skip any sections. Each part of the form is important for your understanding and assessment.
- Do double-check your chemical equations for accuracy. Balancing the equations is key to solving chemistry problems.
- Don't use corrections fluid. If you make a mistake, simply cross it out neatly and write the correction nearby.
- Do take your time on the prior knowledge questions. They set the foundation for the rest of your exploration.
Misconceptions
Here are eight common misconceptions about the Student Chemical Equations form, along with clarifications to help clear them up:
- Misconception 1: A balanced equation means the same number of molecules on both sides.
- Misconception 2: Changing subscripts within a chemical formula is permitted to balance an equation.
- Misconception 3: The law of conservation of matter only applies to reactants in a chemical reaction.
- Misconception 4: Combustion reactions only produce carbon dioxide and water.
- Misconception 5: Subscripts represent the total number of atoms in a molecule.
- Misconception 6: All chemical reactions can be classified into one of the five categories provided.
- Misconception 7: There is only one correct method for balancing equations.
- Misconception 8: The molar mass of a compound is always the same as the sum of its atomic masses.
This is incorrect. A balanced equation requires that the number of each type of atom and not necessarily the number of molecules is the same on both sides of the equation.
This is false. You can only change coefficients (the numbers in front of formulas) to balance an equation. Changing subscripts alters the actual substance being represented.
In reality, the law applies to both reactants and products. No atoms disappear or are created; they are simply rearranged during the reaction.
This is misleading. While carbon dioxide and water are common products of combustion, other substances can form depending on the fuel. For instance, incomplete combustion can yield carbon monoxide.
Not exactly. Subscripts indicate the number of specific atoms of an element within a molecule, not the total number of all atoms present.
This is not always the case. Some reactions may not fit neatly into these categories or may involve traits of multiple types, demonstrating the complexity of chemical interactions.
Actually, there can be multiple ways to arrive at a balanced equation. Different methods might be more convenient based on the specific reaction involved.
This can be misleading. While molar mass does reflect the sum of the atomic masses, it must account for the mass of the molecules and the number of molecules present in one mole of substance.
Key takeaways
Key Takeaways for Filling Out and Using the Student Chemical Equations Form:
- Start by filling in your name and date before moving on to other sections of the form.
- Prior knowledge questions are important. Answer them thoughtfully as they help you understand the concepts before engaging with the Gizmo.
- When balancing an equation, remember that you can only change the number of molecules by adding coefficients; never change the chemical formulas themselves.
- Check the balance of atoms on both sides of the equation frequently to ensure that the law of conservation of matter is satisfied.
Browse Other Templates
Erc Bma - This analysis should be seen as a blueprint for decision-making rather than a mere formality.
Bill of Sale Template Uk - The form effectively outlines the responsibilities to ensure clarity in the sale process.