Fill Out Your Trophon Epr Form
When it comes to ensuring the highest standards of disinfection in healthcare settings, the Trophon EPR form plays a crucial role. This user manual is not just a set of instructions; it’s a vital guide designed to help healthcare professionals navigate the safe and effective use of the Trophon EPR system. The manual covers essential topics, including an overview of the device features, installation guidance, and routine maintenance procedures. In particular, sections within the manual emphasize the importance of proper training for all users to facilitate safe operation and avoid potential hazards associated with disinfectants. It specifies that the Trophon EPR is intended solely for the high-level disinfection of validated ultrasound probes, making it clear that any other applications could compromise safety and effectiveness. For healthcare professionals—whether they are sonographers, nurses, or specialists—recognizing this focused purpose is imperative. Structured to support efficient workflow in varied settings, from hospitals to clinics, the Trophon EPR user manual ensures that the device operates at peak efficiency while complying with necessary health standards. Moreover, specific troubleshooting advice and maintenance tips are included to help users address potential issues quickly to keep operations running smoothly.
Trophon Epr Example







Form Characteristics
| Fact Name | Fact Details |
|---|---|
| Manual Version | Trophon EPR User Manual - USA M00625 2.0, dated June 2011. |
| Intended Use | The Trophon EPR is designed exclusively for high-level disinfection of validated ultrasound probes. |
| Responsible Use | It is the owner’s responsibility to ensure that all users are trained as per the manual instructions. |
| Prohibited Uses | This device should not be used for any applications other than high-level disinfection of ultrasound probes. |
| Disinfectant Compatibility | The Trophon EPR requires the use of Sonex-HL, a high-level instrument grade disinfectant. |
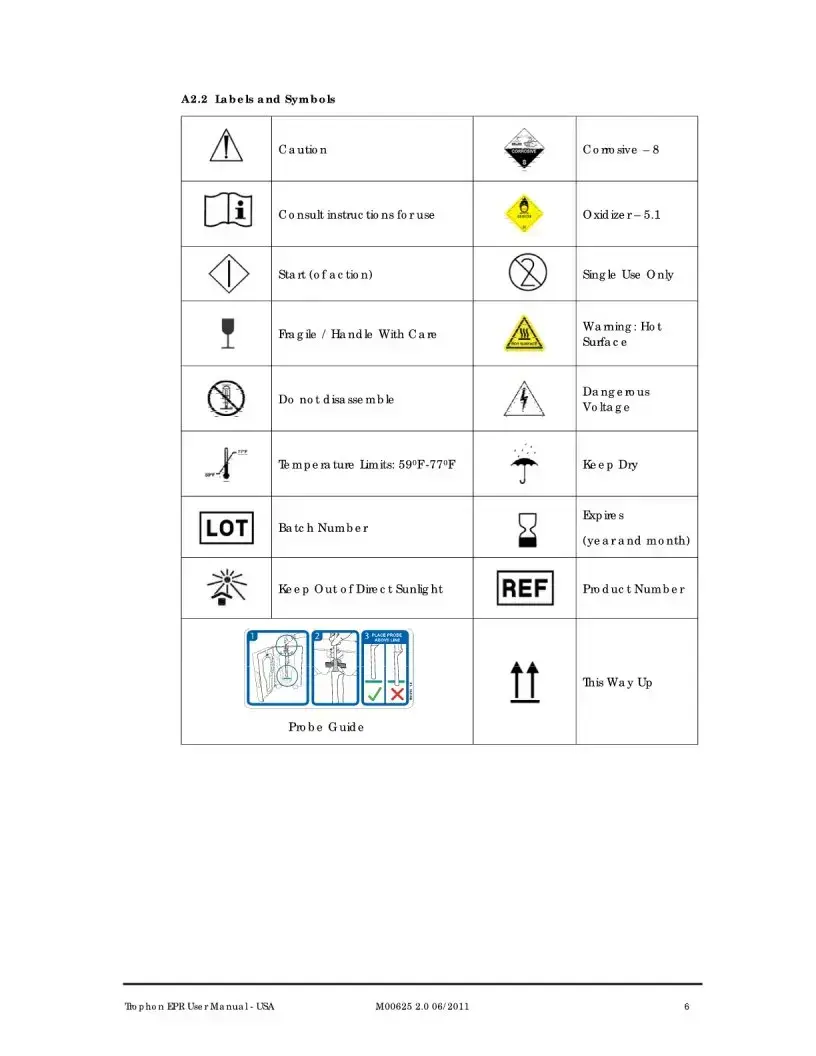
| Labeling and Warnings | Section A2 of the manual includes important warnings, labels, and symbols associated with the device. |
| Manufacturer Information | Manufactured by Nanosonics Limited, located at 3114 N. Grandview Blvd Unit 24, Waukesha, WI 53188, USA. |
Guidelines on Utilizing Trophon Epr
After gathering all necessary information and ensuring the Trophon EPR is set up properly, you will need to complete the Trophon EPR form. This is a vital step that ensures you are following the required procedures for its safe operation. Completing this form accurately contributes to effective and safe disinfection processes.
- First, read the Trophon EPR User Manual to familiarize yourself with operational procedures.
- Gather your business information, such as name, address, and contact details.
- Find the section on the form where you can attach your business card or stamp.
- Fill in the distributor’s information, which, in this case, is GE Healthcare.
- Document the catalog number required, which is E8350ND.
- Ensure all contact information is accurate before submission.
- Review the form for any missing information or inaccuracies.
- Submit the completed form to the designated representative or office.
What You Should Know About This Form
What is the Trophon EPR and what is it used for?
The Trophon EPR is a medical device designed specifically to perform high-level disinfection of validated ultrasound probes. Its purpose is to ensure that these instruments are sanitized adequately before use in healthcare settings. The Trophon EPR is not intended for any other applications, such as reprocessing single-use devices or pre-cleaning ultrasound probes.
Who is responsible for training the users of the Trophon EPR?
It is the responsibility of the device owner to ensure that all users receive proper training according to the instructions provided in the Trophon EPR manual. Training should encompass safe operation, potential hazards related to the disinfectant, and appropriate safety procedures. This diligence helps maintain a safe environment for both patients and medical professionals.
Where can the Trophon EPR be used?
The Trophon EPR is intended for use in various healthcare environments, including hospitals, general practices, and specialized doctor's offices. These locations may have centralized cleaning rooms or operate without specific cleaning facilities. The device is meant to be used by healthcare professionals such as sonographers, nurses, general practitioners, radiographers, and specialists like cardiologists and obstetricians.
What precautions should be taken while using the Trophon EPR?
When using the Trophon EPR, it is crucial to adhere to the manufacturer's guidelines to ensure effective disinfection. Users should be aware of and follow all safety protocols associated with the disinfectant. Regular maintenance and proper loading of disinfectant cartridges are also necessary to achieve intended results. Understanding these precautions helps prevent incomplete or failed disinfection cycles.
What should be done in case of troubleshooting with the Trophon EPR?
If users encounter operational issues, the manual includes a troubleshooting section that provides guidance on addressing common problems. Users should consult this section for advice, or they can contact their customer service representative for assistance. It’s essential to resolve any issues promptly to maintain the device's efficacy and compliance with safety standards.
Common mistakes
Filling out the Trophon EPR form correctly is critical for ensuring proper disinfection processes. However, many people make common mistakes. One frequent error is not including the technician's full name. Failing to provide this information can lead to confusion regarding who performed the disinfection, which is essential for record-keeping and accountability.
Another mistake is neglecting to check that all required boxes are marked. This may seem minor, but unchecked boxes can result in incomplete forms. Incomplete forms may cause delays in processing and could lead to safety risks if the device is not registered correctly. It's vital to diligently review the form to ensure every necessary section is appropriately filled out.
People often rush through the signature section and forget to date their forms. This omission can complicate verification efforts and create questions about when the disinfection occurred. Always remember that both a signature and a date are necessary for validation of the form.
Incomplete information about the environment where the Trophon EPR will be used is another common issue. Users must specify the healthcare setting accurately. Providing incorrect or vague details can lead to misunderstandings about the operational context of the device, which is crucial for compliance with safety standards.
Finally, many fail to attach relevant business cards or stamps as requested. This documentation helps to establish the identity of the technician and their affiliation with the healthcare facility. Not including this information may create a disconnect during follow-up inquiries or inspections.
Documents used along the form
When utilizing the Trophon EPR form, several additional forms and documents may be necessary to ensure proper compliance and operational effectiveness within healthcare settings. Below is a list of those documents, along with a brief description of each.
- User Training Acknowledgment Form: This form is used by healthcare professionals to confirm that they have received proper training on the operation and safety procedures associated with the Trophon EPR.
- Service Report Document: A record that outlines any maintenance or repair work performed on the Trophon EPR, detailing the date, issues addressed, and actions taken.
- Operation Log: This log tracks daily usage of the Trophon EPR, including start and end times, types of procedures performed, and the staff involved.
- Incident Report Form: A standardized form that captures any incidents or near misses related to the operation of the Trophon EPR for quality assurance and improvement purposes.
- Cleaning and Maintenance Checklist: A checklist that ensures routine maintenance tasks are completed, such as cleaning protocols and inspection of the device's components.
- Disinfectant Usage Log: This document records the types and quantities of disinfectants used within the Trophon EPR, ensuring proper inventory management and compliance.
- Warranty Registration Form: A form used to register the Trophon EPR for warranty purposes, including user information and device serial number.
- Compliance Certification: A document that indicates the Trophon EPR meets all necessary regulatory standards, typically required for insurance and accreditation purposes.
- Device Disposal Agreement: A form that outlines procedures for the safe disposal of disinfectant cartridges and ensures compliance with regulatory requirements.
- Manufacturer’s Instructions for Use: A detailed guide provided by the manufacturer, offering specifics on operational guidelines, maintenance, and troubleshooting techniques.
These documents play a critical role in the safe and effective use of the Trophon EPR in healthcare environments. Proper adherence to guidelines and documentation requirements enhances patient safety and care quality.
Similar forms
The Trophon EPR form carries essential information for the use and maintenance of the Trophon EPR disinfection device. It aligns closely with various other medical device documentation, each serving a similar purpose in ensuring safe and effective usage. Here are five documents that share features with the Trophon EPR form:
- Operator's Manual for Medical Equipment: Like the Trophon EPR form, this manual outlines necessary procedures for safe operation, maintenance, and troubleshooting of medical devices. Both documents emphasize the importance of following specific instructions to avoid hazards and ensure efficiency.
- Maintenance Record Form: This form requires users to log routine maintenance and servicing. Similarly, the Trophon EPR form includes maintenance instructions, ensuring users keep accurate records of usage and any necessary upkeep tasks.
- Safety Data Sheet (SDS) for Disinfectants: Just as the Trophon EPR form details the handling of disinfectants, the SDS provides comprehensive safety information about chemical substances, including hazards, handling instructions, and safety precautions.
- Training Manual for Healthcare Professionals: Both the Trophon EPR form and this training manual are designed to ensure that all users receive adequate training. They focus on informing professionals about potential hazards, correct usage protocols, and safe practices related to the equipment.
- Quality Assurance Documentation: This type of document often outlines standards, procedures, and performance metrics for medical devices. The Trophon EPR form includes essential guidelines that align with quality assurance protocols, aimed at achieving effective disinfection and compliance with healthcare regulations.
Dos and Don'ts
When filling out the Trophon EPR form, there are specific actions to take and to avoid to ensure accuracy and compliance with requirements.
- Do: Read the user manual thoroughly before filling out the form.
- Do: Ensure all information entered is accurate and up-to-date.
- Do: Follow all instructions exactly as described in the manual.
- Do: Keep a copy of the completed form for your records.
- Don't: Use any abbreviations that may confuse the intent of the information provided.
- Don't: Skip any sections of the form; all must be completed.
- Don't: Enter information without verifying its correctness first.
- Don't: Assume that previous forms are a reliable source for current entries; always check for changes.
Misconceptions
-
Misconception 1: The Trophon EPR can be used for any type of disinfection.
This is incorrect. The Trophon EPR is specifically designed for high-level disinfection of validated ultrasound probes only. Using it for any other purpose violates its intended use.
-
Misconception 2: It is okay to use the Trophon EPR for single-use devices.
This is not true. The Trophon EPR is not intended for reprocessing single-use devices. Doing so could compromise patient safety and violate regulatory standards.
-
Misconception 3: Operators do not need training to use the Trophon EPR.
This is a dangerous assumption. All users must be trained as per the manual's instructions to ensure safe and effective operation of the device. Lack of training can lead to improper use and ineffective disinfection.
-
Misconception 4: The Trophon EPR can replace the need for cleaning probes before disinfection.
This misconception can lead to serious issues. The Trophon EPR is not intended to pre-clean ultrasound probes. Proper cleaning of probes is essential before using the Trophon EPR to ensure effective disinfection.
Key takeaways
Before using the Trophon EPR, thoroughly read the user manual. This ensures that all procedures are understood and followed properly.
Training is crucial. Users must be educated on operating the device, handling disinfectants safely, and recognizing potential hazards.
Utilize the Trophon EPR specifically for high-level disinfection of validated ultrasound probes. Do not use it for any other purpose.
Keep contact information for your GE Healthcare representative accessible, as they can assist with operational or maintenance queries.
Browse Other Templates
Publx - Your education lays the groundwork for your career pathway.
US-Chile FTA Origin Declaration,Certificate of Authenticity for US-Chile Trade,Origin Verification Document,Free Trade Certificate for US-Chile,Trade Origin Certification Form,US-Chile Tariff Preference Certificate,Certificate of Source for US-Chile - Field 11 requires an authorized signature, affirming the accuracy of the information provided.
Tesco Pet Insurance Uk - Indicate your pet's name and details accurately on the form.
